Cell ( IF 64.5 ) Pub Date : 2021-09-27 , DOI: 10.1016/j.cell.2021.09.005 Lei-Lei Wang 1 , Carolina Serrano 1 , Xiaoling Zhong 1 , Shuaipeng Ma 1 , Yuhua Zou 1 , Chun-Li Zhang 1
|
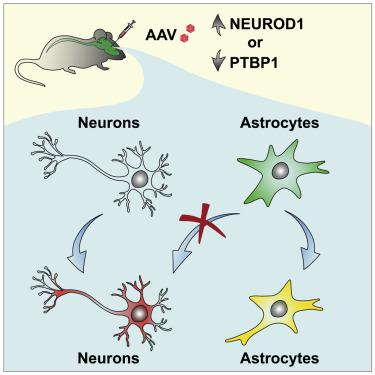
In vivo cell fate conversions have emerged as potential regeneration-based therapeutics for injury and disease. Recent studies reported that ectopic expression or knockdown of certain factors can convert resident astrocytes into functional neurons with high efficiency, region specificity, and precise connectivity. However, using stringent lineage tracing in the mouse brain, we show that the presumed astrocyte-converted neurons are actually endogenous neurons. AAV-mediated co-expression of NEUROD1 and a reporter specifically and efficiently induces reporter-labeled neurons. However, these neurons cannot be traced retrospectively to quiescent or reactive astrocytes using lineage-mapping strategies. Instead, through a retrograde labeling approach, our results reveal that endogenous neurons are the source for these viral-reporter-labeled neurons. Similarly, despite efficient knockdown of PTBP1 in vivo, genetically traced resident astrocytes were not converted into neurons. Together, our results highlight the requirement of lineage-tracing strategies, which should be broadly applied to studies of cell fate conversions in vivo.
中文翻译:

通过体内谱系追踪重新审视星形胶质细胞向神经元的转化
体内细胞命运转换已成为潜在的基于再生的损伤和疾病疗法。最近的研究报道,异位表达或某些因子的敲低可以将驻留的星形胶质细胞转化为具有高效率、区域特异性和精确连接的功能神经元。然而,在小鼠大脑中使用严格的谱系追踪,我们表明假定的星形胶质细胞转化的神经元实际上是内源性神经元。AAV 介导的 NEUROD1 和报告基因的共表达特异性且有效地诱导报告标记的神经元。然而,这些神经元不能使用谱系映射策略追溯追踪到静止或反应性星形胶质细胞。相反,通过逆行标记方法,我们的结果表明内源性神经元是这些病毒报告标记神经元的来源。相似地,在体内,遗传追踪的常驻星形胶质细胞没有转化为神经元。总之,我们的结果强调了谱系追踪策略的要求,该策略应广泛应用于体内细胞命运转换的研究。

























 京公网安备 11010802027423号
京公网安备 11010802027423号