当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Rare Earth Metal Complexes Supported by a Tripodal Tris(amido) Ligand System Featuring an Arene Anchor
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2021-09-27 , DOI: 10.1021/acs.inorgchem.1c01922 Tiansi Xin 1 , Xinrui Wang 1 , Kexin Yang 1 , Jiefeng Liang 1 , Wenliang Huang 1
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2021-09-27 , DOI: 10.1021/acs.inorgchem.1c01922 Tiansi Xin 1 , Xinrui Wang 1 , Kexin Yang 1 , Jiefeng Liang 1 , Wenliang Huang 1
Affiliation
|
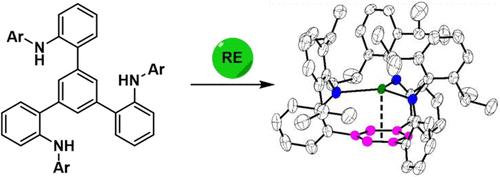
A new tripodal tris(amido) ligand system featuring an arene anchor was developed and applied to the coordination chemistry of rare earth metals. Two tris(amido) ligands with a 1,3,5-triphenylbenzene backbone were prepared in two steps from commercially available reagents on a gram scale. Salt metathesis and alkane elimination reactions were exploited to prepare mononuclear rare earth metal complexes in moderate to good yields. For salt metathesis reactions, while metal tribromides yielded neutral metal tris(amido) complexes, metal trichlorides led to the formation of ate complexes with an additional chloride bound to the metal center. The new compounds were characterized by X-ray crystallography, elemental analysis, and 1H and 13C nuclear magnetic resonance spectroscopy. The rare earth metal complexes exhibit a trigonal planar coordination geometry for the [MN3] fragment in the solid state rather than a trigonal pyramidal geometry, commonly observed for rare earth metal tris(amido) complexes such as M[N(SiMe3)2]3. Moreover, the arene anchor of the tripodal ligands is engaged in a nonnegligible interaction with the rare earth metal ions. Density functional theory calculations were performed to gain insight into the bonding interactions between the tripodal ligands and the rare earth metal ions. While LUMOs of these rare earth metal complexes are mainly π* orbitals of the arene with a minor component of metal-based orbitals, HOMO–15 and HOMO–16 of a lanthanum complex show that the arene anchor serves as a π donor to the trivalent lanthanum ion.
中文翻译:

由具有芳烃锚的三足 Tris(amido) 配体系统支持的稀土金属配合物
开发了一种具有芳烃锚的新型三足三(酰胺)配体系统,并将其应用于稀土金属的配位化学。两个具有 1,3,5-三苯基苯主链的三(酰氨基)配体是从克级市售试剂中分两步制备的。利用盐复分解和烷烃消除反应以中等至良好的收率制备单核稀土金属配合物。对于盐复分解反应,虽然金属三溴化物产生中性金属三(酰氨基)络合物,但金属三氯化物导致形成 ate 络合物,另外的氯化物结合到金属中心。新化合物通过 X 射线晶体学、元素分析和1 H 和13C 核磁共振波谱。稀土金属配合物在固态[MN 3 ] 片段中表现出三角平面配位几何形状,而不是三角锥几何形状,常见于稀土金属三(酰胺)配合物,如 M[N(SiMe 3 ) 2 。 ] 3. 此外,三足配体的芳烃锚与稀土金属离子发生不可忽视的相互作用。进行密度泛函理论计算以深入了解三足配体和稀土金属离子之间的键合相互作用。虽然这些稀土金属配合物的 LUMO 主要是芳烃的 π* 轨道和少量金属基轨道,但镧配合物的 HOMO-15 和 HOMO-16 表明芳烃锚作为三价的 π 供体镧离子。
更新日期:2021-10-18
中文翻译:

由具有芳烃锚的三足 Tris(amido) 配体系统支持的稀土金属配合物
开发了一种具有芳烃锚的新型三足三(酰胺)配体系统,并将其应用于稀土金属的配位化学。两个具有 1,3,5-三苯基苯主链的三(酰氨基)配体是从克级市售试剂中分两步制备的。利用盐复分解和烷烃消除反应以中等至良好的收率制备单核稀土金属配合物。对于盐复分解反应,虽然金属三溴化物产生中性金属三(酰氨基)络合物,但金属三氯化物导致形成 ate 络合物,另外的氯化物结合到金属中心。新化合物通过 X 射线晶体学、元素分析和1 H 和13C 核磁共振波谱。稀土金属配合物在固态[MN 3 ] 片段中表现出三角平面配位几何形状,而不是三角锥几何形状,常见于稀土金属三(酰胺)配合物,如 M[N(SiMe 3 ) 2 。 ] 3. 此外,三足配体的芳烃锚与稀土金属离子发生不可忽视的相互作用。进行密度泛函理论计算以深入了解三足配体和稀土金属离子之间的键合相互作用。虽然这些稀土金属配合物的 LUMO 主要是芳烃的 π* 轨道和少量金属基轨道,但镧配合物的 HOMO-15 和 HOMO-16 表明芳烃锚作为三价的 π 供体镧离子。


























 京公网安备 11010802027423号
京公网安备 11010802027423号