当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Total Syntheses of Hikosamine and Hikizimycin
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2021-09-27 , DOI: 10.1021/acs.joc.1c01773 Haruka Fujino 1 , Masanori Nagatomo 1 , Masayuki Inoue 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2021-09-27 , DOI: 10.1021/acs.joc.1c01773 Haruka Fujino 1 , Masanori Nagatomo 1 , Masayuki Inoue 1
Affiliation
|
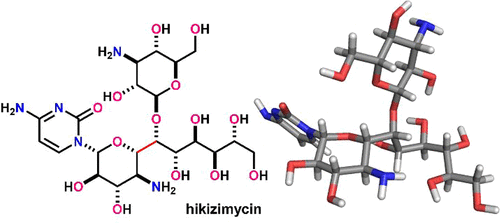
Hikizimycin (1) is a potent anthelmintic and antibacterial natural product. The core 4-amino-4-deoxyundecose sugar (hikosamine) of 1 consists of an 11-carbon linear chain substituted with one amino group and 10 hydroxy groups. The C1 and C6O positions of the 10 contiguous stereocenters are further appended by a cytosine base and a 3-amino-3-deoxyglucose sugar (kanosamine), respectively. Since the structural determination in the early 1970s, synthetic chemists have been attracted by this exceedingly complex structure and have investigated the full chemical construction of 1. These synthetic efforts culminated in four syntheses of the protected hikosamines and two total syntheses of 1. In this Perspective, we summarize the strategies and tactics utilized in these syntheses to showcase the evolution of modern natural product synthesis.
中文翻译:

Hikosamine和Hikizimycin的全合成
Hikizimycin ( 1 ) 是一种有效的驱虫和抗菌天然产物。1的核心4-氨基-4-脱氧十一糖(hikosamine)由一个11个碳的直链组成,被一个氨基和10个羟基取代。10 个连续立体中心的 C1 和 C6O 位置分别进一步附加了一个胞嘧啶碱基和一个 3-氨基-3-脱氧葡萄糖(卡诺胺)。自 1970 年代初的结构确定以来,合成化学家就被这种极其复杂的结构所吸引,并研究了1的完整化学结构。这些合成努力最终导致了受保护的 hikosamines 的四种合成和1的两种总合成。. 在这个观点中,我们总结了这些合成中使用的策略和策略,以展示现代天然产物合成的演变。
更新日期:2021-12-03
中文翻译:

Hikosamine和Hikizimycin的全合成
Hikizimycin ( 1 ) 是一种有效的驱虫和抗菌天然产物。1的核心4-氨基-4-脱氧十一糖(hikosamine)由一个11个碳的直链组成,被一个氨基和10个羟基取代。10 个连续立体中心的 C1 和 C6O 位置分别进一步附加了一个胞嘧啶碱基和一个 3-氨基-3-脱氧葡萄糖(卡诺胺)。自 1970 年代初的结构确定以来,合成化学家就被这种极其复杂的结构所吸引,并研究了1的完整化学结构。这些合成努力最终导致了受保护的 hikosamines 的四种合成和1的两种总合成。. 在这个观点中,我们总结了这些合成中使用的策略和策略,以展示现代天然产物合成的演变。


























 京公网安备 11010802027423号
京公网安备 11010802027423号