Journal of Molecular Liquids ( IF 6 ) Pub Date : 2021-09-27 , DOI: 10.1016/j.molliq.2021.117663 Jia Wang 1 , Jinyan Liu 1 , Qian Liu 1 , Yao Chong 1
|
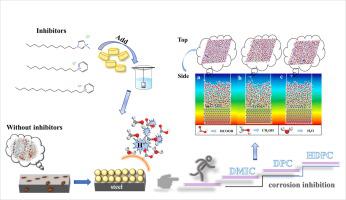
The present paper discusses the adsorption and inhibitory effect of nitrogen-containing compound, 1-dodecyl-3-methyimidazolium chloride (DMIC), dodecylpyridinium chloride (DPC) and hexadecylpyridinium chloride monohydrate (HDPC) on Q235 steel corrosion in a new system(10 mol·L−1 methanol/0.1 mol·L−1 formic acid medium. The experimental study was carried out using a series of techniques such as weight loss, potentiodynamic polarization (PDP) and electrochemical impedance spectroscopy (EIS). contact angle test, Raman spectra, X-ray photoelectron spectroscopy (XPS) and atomic force microscope (AFM) served to identify microscopic variation on Fe surface. Adsorption isotherm model controlling the adsorption process are calculated and discussed. Moreover, the relationships between molecular structure and corrosion inhibition performance of three inhibitors were verified by quantum chemical calculation and molecular dynamics simulation. The results obtained from the electrochemical methods showed that DMIC, DPC and HDPC imparted high resistance and behaved as mixed-type corrosion inhibitors dominated with anode. inhibition efficiency (η%) increased with the increase of inhibitors concentration to attained 88.81%, 92.69%, 93.67% at 0.06 mol·L−1, respectively. Surface studies confirmed the existence of an adsorption film on the metal surface. The obtained results demonstrated that the adsorption process on the metal surface followed the Langmuir adsorption model. Quantum chemical calculations and molecular dynamics simulation (MD) testified the effect of the chemical structure of three inhibitors on its inhibition efficiency, which had a good agreement with the experimental data.
中文翻译:

甲醇/甲酸介质中杂环化合物对Q235钢的缓蚀性能:实验与理论
本文讨论了含氮化合物、1-十二烷基-3-甲基咪唑氯化物(DMIC)、十二烷基氯化吡啶鎓(DPC)和十六烷基氯化吡啶鎓(HDPC)在新体系(10 mol)中对Q235钢腐蚀的吸附和抑制作用。 ·L -1甲醇/0.1 mol·L -1甲酸介质。实验研究使用了一系列技术,如失重、动电位极化 (PDP) 和电化学阻抗谱 (EIS)。接触角测试、拉曼光谱、X 射线光电子能谱 (XPS) 和原子力显微镜 (AFM) 用于识别 Fe 表面的微观变化。计算和讨论了控制吸附过程的吸附等温线模型。并通过量子化学计算和分子动力学模拟验证了三种缓蚀剂的分子结构与缓蚀性能之间的关系。从电化学方法获得的结果表明,DMIC、DPC 和 HDPC 具有高电阻并表现为以阳极为主的混合型缓蚀剂。抑制效率(η %)随着抑制剂浓度的增加而增加,在0.06 mol·L -1时分别达到88.81%、92.69%、93.67% 。表面研究证实金属表面存在吸附膜。所得结果表明金属表面的吸附过程遵循朗缪尔吸附模型。量子化学计算和分子动力学模拟(MD)验证了三种抑制剂的化学结构对其抑制效率的影响,与实验数据吻合较好。



























 京公网安备 11010802027423号
京公网安备 11010802027423号