International Journal of Hydrogen Energy ( IF 7.2 ) Pub Date : 2021-09-27 , DOI: 10.1016/j.ijhydene.2021.09.050 Haitao Wang 1 , Xin Jiao 1 , Wenlu Zeng 1 , Ying Zhang 1 , Yongli Jiao 1
|
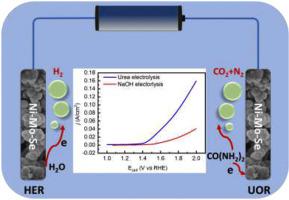
Electrodeposition provides a simple but effective way to prepare advanced electrode for the application in electrochemical field. In this work, NiMoSe ternary nanospheres were deposited on nickel foam (NiMoSe/NF) by one-step electrodeposition. The morphology, phase and chemical composition of the electrode was characterized by using SEM, TEM, XRD and XPS. The electrode exhibited excellent performance for both urea oxidation reaction (UOR) and hydrogen evolution reaction (HER). It only required 1.39 V and 81 mV (vs. RHE) to deliver a current density of 10 mA/cm2 for UOR and HER, respectively. The electrolyzer constructed with NiMoSe/NF as both anode and cathode could deliver a current density of 10 mA/cm2 at a driving potential of 1.44 V. The stability test showed that the electrode had good durability as electrode for both UOR and HER. Considering the easiness, simplicity and low cost, the NiMoSe/NF electrode could find wide application in urea electrolysis.
中文翻译:

在泡沫镍上电沉积 NiMoSe 三元纳米球作为尿素电解和析氢反应的双功能电催化剂
电沉积为制备用于电化学领域的先进电极提供了一种简单而有效的方法。在这项工作中,NiMoSe 三元纳米球通过一步电沉积沉积在镍泡沫 (NiMoSe/NF) 上。采用SEM、TEM、XRD和XPS对电极的形貌、物相和化学成分进行表征。该电极在尿素氧化反应 (UOR) 和析氢反应 (HER) 中均表现出优异的性能。分别为 UOR 和 HER提供 10 mA/cm 2的电流密度只需要 1.39 V 和 81 mV(相对于 RHE)。以 NiMoSe/NF 作为阳极和阴极构建的电解槽可以提供 10 mA/cm 2的电流密度在 1.44 V 的驱动电位下。稳定性测试表明,该电极作为 UOR 和 HER 的电极具有良好的耐久性。NiMoSe/NF 电极具有操作简便、成本低等优点,可广泛应用于尿素电解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号