当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
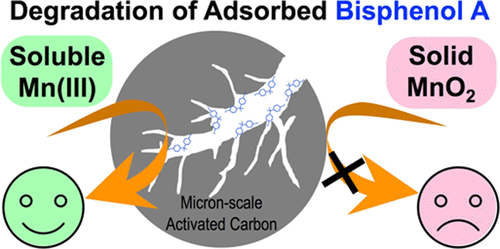
Degradation of Adsorbed Bisphenol A by Soluble Mn(III)
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2021-09-24 , DOI: 10.1021/acs.est.1c03862 Yanchen Sun 1, 2, 3 , Jeongdae Im 4 , Nusrat Shobnam 4 , Sofia K Fanourakis 5 , Lilin He 6 , Lawrence M Anovitz 7 , Paul R Erickson 8 , Huihui Sun 9 , Jie Zhuang 9 , Frank E Löffler 1, 2, 3, 9, 10
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2021-09-24 , DOI: 10.1021/acs.est.1c03862 Yanchen Sun 1, 2, 3 , Jeongdae Im 4 , Nusrat Shobnam 4 , Sofia K Fanourakis 5 , Lilin He 6 , Lawrence M Anovitz 7 , Paul R Erickson 8 , Huihui Sun 9 , Jie Zhuang 9 , Frank E Löffler 1, 2, 3, 9, 10
Affiliation
|
Bisphenol A (BPA), a high production volume chemical and potential endocrine disruptor, is found to be associated with sediments and soils due to its hydrophobicity (log KOW of 3.42). We used superfine powdered activated carbon (SPAC) with a particle size of 1.38 ± 0.03 μm as a BPA sorbent and assessed degradation of BPA by oxidized manganese (Mn) species. SPAC strongly sorbed BPA, and desorption required organic solvents. No degradation of adsorbed BPA (278.7 ± 0.6 mg BPA g–1 SPAC) was observed with synthetic, solid α-MnO2 with a particle size of 15.41 ± 1.35 μm; however, 89% mass reduction occurred following the addition of 0.5 mM soluble Mn(III). Small-angle neutron scattering data suggested that both adsorption and degradation of BPA occurred in SPAC pores. The findings demonstrate that Mn(III) mediates oxidative transformation of dissolved and adsorbed BPA, the latter observation challenging the paradigm that contaminant desorption and diffusion out of pore structures are required steps for degradation. Soluble Mn(III) is abundant near oxic-anoxic interfaces, and the observation that adsorbed BPA is susceptible to degradation has implications for predicting, and possibly managing, the fate and longevity of BPA in environmental systems.
中文翻译:

可溶性Mn(III)降解吸附双酚A
双酚 A (BPA) 是一种高产量的化学物质和潜在的内分泌干扰物,由于其疏水性(log K OW为 3.42),被发现与沉积物和土壤有关。我们使用粒径为 1.38 ± 0.03 μm 的超细粉状活性炭 (SPAC) 作为 BPA 吸附剂,并评估了氧化锰 (Mn) 物质对 BPA 的降解。SPAC 强烈吸附 BPA,解吸需要有机溶剂。使用合成的固体 α-MnO 2未观察到吸附的 BPA(278.7 ± 0.6 mg BPA g –1 SPAC)降解粒径为 15.41 ± 1.35 μm;然而,在添加 0.5 mM 可溶性 Mn(III) 后,质量减少了 89%。小角度中子散射数据表明 BPA 的吸附和降解都发生在 SPAC 孔隙中。研究结果表明,Mn(III) 介导溶解和吸附的 BPA 的氧化转化,后者的观察挑战了污染物解吸和扩散出孔结构是降解所需步骤的范式。可溶性 Mn(III) 在好氧-缺氧界面附近含量丰富,吸附的 BPA 易于降解的观察结果对预测和可能管理 BPA 在环境系统中的命运和寿命具有重要意义。
更新日期:2021-10-06
中文翻译:

可溶性Mn(III)降解吸附双酚A
双酚 A (BPA) 是一种高产量的化学物质和潜在的内分泌干扰物,由于其疏水性(log K OW为 3.42),被发现与沉积物和土壤有关。我们使用粒径为 1.38 ± 0.03 μm 的超细粉状活性炭 (SPAC) 作为 BPA 吸附剂,并评估了氧化锰 (Mn) 物质对 BPA 的降解。SPAC 强烈吸附 BPA,解吸需要有机溶剂。使用合成的固体 α-MnO 2未观察到吸附的 BPA(278.7 ± 0.6 mg BPA g –1 SPAC)降解粒径为 15.41 ± 1.35 μm;然而,在添加 0.5 mM 可溶性 Mn(III) 后,质量减少了 89%。小角度中子散射数据表明 BPA 的吸附和降解都发生在 SPAC 孔隙中。研究结果表明,Mn(III) 介导溶解和吸附的 BPA 的氧化转化,后者的观察挑战了污染物解吸和扩散出孔结构是降解所需步骤的范式。可溶性 Mn(III) 在好氧-缺氧界面附近含量丰富,吸附的 BPA 易于降解的观察结果对预测和可能管理 BPA 在环境系统中的命运和寿命具有重要意义。


























 京公网安备 11010802027423号
京公网安备 11010802027423号