Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Stabilizing Role of Water Solvation on Anion−π Interactions in Proteins
ACS Omega ( IF 4.1 ) Pub Date : 2021-09-23 , DOI: 10.1021/acsomega.1c03264 Kanagasabai Balamurugan 1 , M Teresa Pisabarro 1
ACS Omega ( IF 4.1 ) Pub Date : 2021-09-23 , DOI: 10.1021/acsomega.1c03264 Kanagasabai Balamurugan 1 , M Teresa Pisabarro 1
Affiliation
|
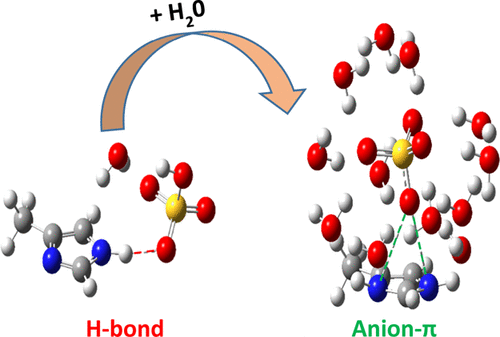
In this work, anion−π interactions between sulfate groups (SO42–) and protein aromatic amino acids (AAs) (histidine protonated (HisP), histidine neutral (HisN), tyrosine (Tyr), tryptophan (Trp), and phenylalanine (Phe)) in an aqueous environment have been analyzed using quantum chemical (QC) calculations and molecular dynamics (MD) simulations. Sulfates can occur naturally in solution and can be contained in biomolecules playing relevant roles in their biological function. In particular, the presence of sulfate groups in glycosaminoglycans such as heparin and heparan sulfate has been shown to be relevant for protein and cellular communication and, consequently, for tissue regeneration. Therefore, anion−π interactions between sulfate groups and aromatic residues represent a relevant aspect to investigate. QC results show that such an anion−π mode of interaction between SO42– and aromatic AAs is only possible in the presence of water molecules, in the absence of any other cooperative non-covalent interactions. Protonated histidine stands out in terms of its enhancement in the magnitude of interaction strength on solvation. Other AAs such as non-protonated histidine, tyrosine, and phenylalanine can stabilize anion−π interactions on solvation, albeit with weak interaction energy. Tryptophan does not exhibit any anion−π mode of interaction with SO42–. The order of magnitude of the interaction of aromatic AAs with SO42– on microsolvation is HisP > HisN > Tyr > Trp > Phe. Atoms in molecules (AIM) analysis illustrates the significance of water molecules in stabilizing the divalent SO42– anion over the π surface of the aromatic AAs. MD simulation analysis shows that the order of magnitude of the interaction of SO42– with aromatic AAs in macroscopic solvation is HisP > HisN, Tyr, Trp > Phe, which is very much in line with the QC results. Spatial distribution function analysis illustrates that protonated histidine alone is capable of establishing the anion−π interaction with SO42– in the solution phase. This study sheds light on the understanding of anion−π interactions between SO42– and aromatic AAs such as His and Tyr observed in protein crystal structures and the significance of water molecules in stabilizing such interactions, which is not feasible otherwise.
中文翻译:

水溶剂化对蛋白质中阴离子-π相互作用的稳定作用
在这项工作中,硫酸根 (SO 4 2–) 和蛋白质芳香族氨基酸 (AAs)(组氨酸质子化 (HisP)、组氨酸中性 (HisN)、酪氨酸 (Tyr)、色氨酸 (Trp) 和苯丙氨酸 (Phe))在水性环境中已使用量子化学 (QC) 进行了分析) 计算和分子动力学 (MD) 模拟。硫酸盐可以在溶液中自然存在,并且可以包含在在其生物学功能中发挥相关作用的生物分子中。特别是,糖胺聚糖(如肝素和硫酸乙酰肝素)中硫酸根的存在已被证明与蛋白质和细胞通讯有关,因此与组织再生有关。因此,硫酸根基团和芳香族残基之间的阴离子-π 相互作用代表了一个需要研究的相关方面。QC 结果表明,SO 4之间的这种阴离子-π 相互作用模式2-和芳香族 AA 仅在存在水分子的情况下才有可能,而没有任何其他协同的非共价相互作用。质子化组氨酸在溶剂化相互作用强度的增强方面脱颖而出。其他 AA 如非质子化组氨酸、酪氨酸和苯丙氨酸可以稳定溶剂化时的阴离子-π 相互作用,尽管相互作用能较弱。色氨酸不表现出任何与 SO 4 2-相互作用的阴离子-π 模式。芳香族 AA 与 SO 4 2–在微溶剂化中相互作用的数量级为 HisP > HisN > Tyr > Trp > Phe。分子中的原子 (AIM) 分析说明了水分子在稳定二价 SO 4 2– 方面的重要性芳香族 AA 的 π 表面上的阴离子。MD模拟分析表明,宏观溶剂化中SO 4 2–与芳香族AA相互作用的数量级为HisP>HisN、Tyr、Trp>Phe,这与QC结果非常吻合。空间分布函数分析表明,单独的质子化组氨酸能够在溶液相中建立阴离子-π 与 SO 4 2- 的相互作用。这项研究阐明了对 SO 4 2-与芳香族 AA(如在蛋白质晶体结构中观察到的 His 和 Tyr)之间的阴离子-π 相互作用的理解,以及水分子在稳定这种相互作用中的重要性,否则这是不可行的。
更新日期:2021-10-06
中文翻译:

水溶剂化对蛋白质中阴离子-π相互作用的稳定作用
在这项工作中,硫酸根 (SO 4 2–) 和蛋白质芳香族氨基酸 (AAs)(组氨酸质子化 (HisP)、组氨酸中性 (HisN)、酪氨酸 (Tyr)、色氨酸 (Trp) 和苯丙氨酸 (Phe))在水性环境中已使用量子化学 (QC) 进行了分析) 计算和分子动力学 (MD) 模拟。硫酸盐可以在溶液中自然存在,并且可以包含在在其生物学功能中发挥相关作用的生物分子中。特别是,糖胺聚糖(如肝素和硫酸乙酰肝素)中硫酸根的存在已被证明与蛋白质和细胞通讯有关,因此与组织再生有关。因此,硫酸根基团和芳香族残基之间的阴离子-π 相互作用代表了一个需要研究的相关方面。QC 结果表明,SO 4之间的这种阴离子-π 相互作用模式2-和芳香族 AA 仅在存在水分子的情况下才有可能,而没有任何其他协同的非共价相互作用。质子化组氨酸在溶剂化相互作用强度的增强方面脱颖而出。其他 AA 如非质子化组氨酸、酪氨酸和苯丙氨酸可以稳定溶剂化时的阴离子-π 相互作用,尽管相互作用能较弱。色氨酸不表现出任何与 SO 4 2-相互作用的阴离子-π 模式。芳香族 AA 与 SO 4 2–在微溶剂化中相互作用的数量级为 HisP > HisN > Tyr > Trp > Phe。分子中的原子 (AIM) 分析说明了水分子在稳定二价 SO 4 2– 方面的重要性芳香族 AA 的 π 表面上的阴离子。MD模拟分析表明,宏观溶剂化中SO 4 2–与芳香族AA相互作用的数量级为HisP>HisN、Tyr、Trp>Phe,这与QC结果非常吻合。空间分布函数分析表明,单独的质子化组氨酸能够在溶液相中建立阴离子-π 与 SO 4 2- 的相互作用。这项研究阐明了对 SO 4 2-与芳香族 AA(如在蛋白质晶体结构中观察到的 His 和 Tyr)之间的阴离子-π 相互作用的理解,以及水分子在稳定这种相互作用中的重要性,否则这是不可行的。


























 京公网安备 11010802027423号
京公网安备 11010802027423号