当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis and Optical Properties of Thiazolo-Chlorin and Porphyrin Skeletons
Organic Letters ( IF 5.2 ) Pub Date : 2021-09-24 , DOI: 10.1021/acs.orglett.1c03001 Takeo Nakano 1, 2, 3 , Hiroaki Imoto 4 , Kensuke Naka 1, 4
Organic Letters ( IF 5.2 ) Pub Date : 2021-09-24 , DOI: 10.1021/acs.orglett.1c03001 Takeo Nakano 1, 2, 3 , Hiroaki Imoto 4 , Kensuke Naka 1, 4
Affiliation
|
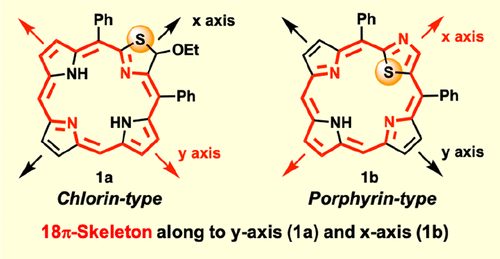
Macrocyclic π-skeletons containing a thiazole moiety were synthesized via MacDonald [3 + 1]-type condensation. The construction of thiazolochlorin 1a and thiazoloporphyrin 1b depended on the conformation of the thiazole moieties, and their 18π-systems expanded along the molecular y and x axes, respectively. In particular, the structure of thiazolochlorin 1a was studied in detail using 2D nuclear magnetic resonance methods. The optical properties in solution were measured and discussed based on both experimental data and computational studies.
中文翻译:

噻唑啉和卟啉骨架的合成和光学性质
含有噻唑部分的大环 π 骨架是通过 MacDonald [3 + 1] 型缩合合成的。噻唑并二氢卟啉1a和噻唑并卟啉1b的构建取决于噻唑部分的构象,它们的 18π 系统分别沿分子y和x轴扩展。特别是,使用二维核磁共振方法详细研究了噻唑并二氢卟吩1a的结构。基于实验数据和计算研究测量和讨论了溶液中的光学特性。
更新日期:2021-10-15
中文翻译:

噻唑啉和卟啉骨架的合成和光学性质
含有噻唑部分的大环 π 骨架是通过 MacDonald [3 + 1] 型缩合合成的。噻唑并二氢卟啉1a和噻唑并卟啉1b的构建取决于噻唑部分的构象,它们的 18π 系统分别沿分子y和x轴扩展。特别是,使用二维核磁共振方法详细研究了噻唑并二氢卟吩1a的结构。基于实验数据和计算研究测量和讨论了溶液中的光学特性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号