当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Coinage metal aluminyl complexes: probing regiochemistry and mechanism in the insertion and reduction of carbon dioxide
Chemical Science ( IF 8.4 ) Pub Date : 2021-09-16 , DOI: 10.1039/d1sc04676d Caitilín McManus 1 , Jamie Hicks 1 , Xianlu Cui 2 , Lili Zhao 2 , Gernot Frenking 3 , Jose M Goicoechea 1 , Simon Aldridge 1
Chemical Science ( IF 8.4 ) Pub Date : 2021-09-16 , DOI: 10.1039/d1sc04676d Caitilín McManus 1 , Jamie Hicks 1 , Xianlu Cui 2 , Lili Zhao 2 , Gernot Frenking 3 , Jose M Goicoechea 1 , Simon Aldridge 1
Affiliation
|
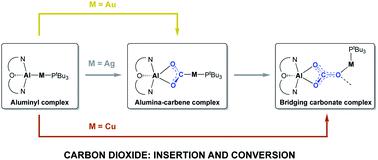
The synthesis of coinage metal aluminyl complexes, featuring M–Al covalent bonds, is reported via a salt metathesis approach employing an anionic Al(I) (‘aluminyl’) nucleophile and group 11 electrophiles. This approach allows access to both bimetallic (1 : 1) systems of the type (tBu3P)MAl(NON) (M = Cu, Ag, Au; NON = 4,5-bis(2,6-diisopropylanilido)-2,7-di-tert-butyl-9,9-dimethylxanthene) and a 2 : 1 di(aluminyl)cuprate system, K[Cu{Al(NON)}2]. The bimetallic complexes readily insert heteroallenes (CO2, carbodiimides) into the unsupported M–Al bonds to give systems containing a M(CE2)Al bridging unit (E = O, NR), with the μ-κ1(C):κ2(E,E′) mode of heteroallene binding being demonstrated crystallographically for carbodiimide insertion in the cases of all three metals, Cu, Ag and Au. The regiochemistry of these processes, leading to the formation of M–C bonds, is rationalized computationally, and is consistent with addition of CO2 across the M–Al covalent bond with the group 11 metal acting as the nucleophilic partner and Al as the electrophile. While the products of carbodiimide insertion are stable to further reaction, their CO2 analogues have the potential to react further, depending on the identity of the group 11 metal. (tBu3P)Au(CO)2Al(NON) is inert to further reaction, but its silver counterpart reacts slowly with CO2 to give the corresponding carbonate complex (and CO), and the copper system proceeds rapidly to the carbonate even at low temperatures. Experimental and quantum chemical investigations of the mechanism of the CO2 to CO/carbonate transformation are consistent with rate-determining extrusion of CO from the initially-formed M(CO)2Al fragment to give a bimetallic oxide that rapidly assimilates a second molecule of CO2. The calculated energetic barriers for the most feasible CO extrusion step (ΔG‡ = 26.6, 33.1, 44.5 kcal mol−1 for M = Cu, Ag and Au, respectively) are consistent not only with the observed experimental labilities of the respective M(CO)2Al motifs, but also with the opposing trends in M–C (increasing) and M–O bond strengths (decreasing) on transitioning from Cu to Au.
中文翻译:

造币金属铝基配合物:探索二氧化碳插入和还原的区域化学和机制
通过盐复分解方法使用阴离子 Al( I )(“铝基”)亲核试剂和第 11 族亲电试剂合成具有 M-Al 共价键的铸币金属铝基配合物。这种方法允许使用 ( t Bu 3 P)MAl(NON) (M = Cu、Ag、Au;NON = 4,5-双(2,6-二异丙基苯胺)-类型的双金属 (1:1) 系统2,7-二叔丁基-9,9-二甲基呫吨)和 2:1 二(铝基)铜酸盐体系,K[Cu{Al(NON)} 2 ]。双金属配合物很容易将杂丙二烯(CO 2,碳二亚胺)插入无载体的 M-Al 键中,得到含有 M(CE 2 )Al 桥接单元(E = O,NR)的体系,其中 μ-κ图 1 (C):κ 2 (E,E') 杂丙二烯键合模式在所有三种金属 Cu、Ag 和 Au 的情况下通过晶体学证明碳二亚胺插入。这些过程的区域化学导致 M-C 键的形成,在计算上是合理的,并且与在 M-Al 共价键上添加 CO 2一致,第 11 族金属作为亲核伙伴,Al 作为亲电试剂. 虽然碳二亚胺插入的产物对进一步反应稳定,但它们的 CO 2类似物有可能进一步反应,具体取决于第 11 族金属的特性。( t Bu 3 P)Au(CO) 2Al(NON) 对进一步反应呈惰性,但其银对应物与 CO 2缓慢反应生成相应的碳酸盐配合物(和 CO),即使在低温下,铜体系也能快速生成碳酸盐。CO 2到 CO/碳酸盐转化机制的实验和量子化学研究与从最初形成的 M(CO) 2 Al 碎片中挤出 CO 的速率决定性挤出一致,得到双金属氧化物,该氧化物可快速同化第二个分子一氧化碳2。最可行的 CO 挤出步骤的计算能量势垒 (Δ G ‡ = 26.6, 33.1, 44.5 kcal mol -1对于 M = Cu、Ag 和 Au,分别)不仅与观察到的各个 M(CO) 2 Al 基序的实验不稳定性一致,而且与 M-C(增加)和 M-O 键强度的相反趋势一致(减少)从 Cu 过渡到 Au。
更新日期:2021-09-24
中文翻译:

造币金属铝基配合物:探索二氧化碳插入和还原的区域化学和机制
通过盐复分解方法使用阴离子 Al( I )(“铝基”)亲核试剂和第 11 族亲电试剂合成具有 M-Al 共价键的铸币金属铝基配合物。这种方法允许使用 ( t Bu 3 P)MAl(NON) (M = Cu、Ag、Au;NON = 4,5-双(2,6-二异丙基苯胺)-类型的双金属 (1:1) 系统2,7-二叔丁基-9,9-二甲基呫吨)和 2:1 二(铝基)铜酸盐体系,K[Cu{Al(NON)} 2 ]。双金属配合物很容易将杂丙二烯(CO 2,碳二亚胺)插入无载体的 M-Al 键中,得到含有 M(CE 2 )Al 桥接单元(E = O,NR)的体系,其中 μ-κ图 1 (C):κ 2 (E,E') 杂丙二烯键合模式在所有三种金属 Cu、Ag 和 Au 的情况下通过晶体学证明碳二亚胺插入。这些过程的区域化学导致 M-C 键的形成,在计算上是合理的,并且与在 M-Al 共价键上添加 CO 2一致,第 11 族金属作为亲核伙伴,Al 作为亲电试剂. 虽然碳二亚胺插入的产物对进一步反应稳定,但它们的 CO 2类似物有可能进一步反应,具体取决于第 11 族金属的特性。( t Bu 3 P)Au(CO) 2Al(NON) 对进一步反应呈惰性,但其银对应物与 CO 2缓慢反应生成相应的碳酸盐配合物(和 CO),即使在低温下,铜体系也能快速生成碳酸盐。CO 2到 CO/碳酸盐转化机制的实验和量子化学研究与从最初形成的 M(CO) 2 Al 碎片中挤出 CO 的速率决定性挤出一致,得到双金属氧化物,该氧化物可快速同化第二个分子一氧化碳2。最可行的 CO 挤出步骤的计算能量势垒 (Δ G ‡ = 26.6, 33.1, 44.5 kcal mol -1对于 M = Cu、Ag 和 Au,分别)不仅与观察到的各个 M(CO) 2 Al 基序的实验不稳定性一致,而且与 M-C(增加)和 M-O 键强度的相反趋势一致(减少)从 Cu 过渡到 Au。



























 京公网安备 11010802027423号
京公网安备 11010802027423号