当前位置:
X-MOL 学术
›
Photochem. Photobiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ability of the Putative Decomposition Products of 2,3-dioxetanes of Indoles to Photosensitize Cyclobutane Pyrimidine Dimer (CPD) Formation and its Implications for the “Dark” (Chemisensitized) Pathway to CPDs in Melanocytes†
Photochemistry and Photobiology ( IF 3.3 ) Pub Date : 2021-09-24 , DOI: 10.1111/php.13529 Yanjing Wang 1 , Elena M Cacchillo 1 , Dariusz M Niedzwiedzki 2, 3 , John-Stephen Taylor 1
Photochemistry and Photobiology ( IF 3.3 ) Pub Date : 2021-09-24 , DOI: 10.1111/php.13529 Yanjing Wang 1 , Elena M Cacchillo 1 , Dariusz M Niedzwiedzki 2, 3 , John-Stephen Taylor 1
Affiliation
|
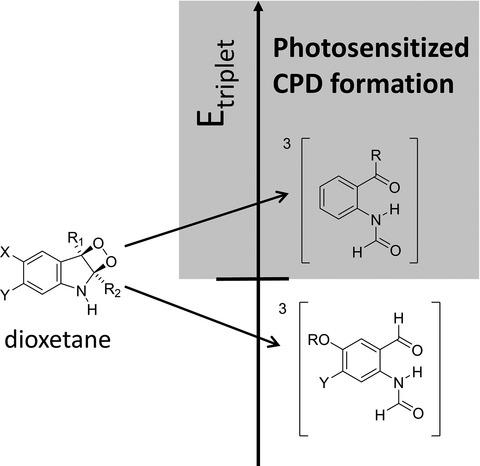
The formation of cyclobutane pyrimidine dimers (CPDs) by a “dark” pathway in melanocytes has been attributed to chemisensitization by dioxetanes produced from peroxynitrite oxidation of melanin or melanin precursors. These dioxetanes are proposed to decompose to triplet state compounds which sensitize CPD formation by triplet–triplet energy transfer. To determine whether such compounds are capable of sensitizing CPD formation, the putative decomposition products of 2,3-dioxetanes of variously substituted indoles were synthesized and their triplet state energies determined at 77 K. Their ability to photosensitize CPD formation was determined by an enzyme-coupled gel electrophoresis assay in comparison with norfloxacin (NFX) which has the lowest triplet energy known to sensitize CPD formation. The decomposition products of 2,3-dioxetanes of 5-hydroxy and 5,6-dimethoxy indoles used as models for melanin precursors had lower triplet energies and were incapable of photosensitizing CPD formation. Theoretical calculations suggest that the decomposition products of the 2,3-dioxetanes of melanin precursors DHI and DHICA will have similarly low triplet energies. Decomposition products of the 2,3-dioxetanes of indoles lacking oxygen substituents had higher triplet energies than NFX and were capable of photosensitizing CPD formation, suggesting that peroxynitrite oxidation of tryptophan could play a hitherto unrecognized role in the dark pathway to CPDs.
中文翻译:

吲哚的 2,3-二氧杂环丁烷的假定分解产物对环丁烷嘧啶二聚体 (CPD) 形成的光敏能力及其对黑素细胞中 CPD 的“暗”(化学致敏)途径的影响†
通过黑色素细胞中的“暗”途径形成环丁烷嘧啶二聚体 (CPD) 归因于黑色素或黑色素前体的过氧亚硝酸盐氧化产生的二氧杂环丁烷的化学敏化作用。建议这些二氧杂环丁烷分解成三重态化合物,通过三重态-三重态能量转移使 CPD 形成敏感。为了确定这些化合物是否能够敏化 CPD 的形成,合成了各种取代的吲哚的 2,3-二氧杂环丁烷的假定分解产物,并在 77 K 下测定了它们的三重态能量。它们对 CPD 形成的光敏化能力由酶确定。与具有最低三重态能量的诺氟沙星 (NFX) 相比,偶联凝胶电泳分析对 CPD 形成敏感。2的分解产物,用作黑色素前体模型的 5-羟基和 5,6-二甲氧基吲哚的 3-二氧杂环丁烷具有较低的三重态能量并且不能形成光敏 CPD。理论计算表明,黑色素前体 DHI 和 DHICA 的 2,3-二氧杂环丁烷的分解产物将具有类似的低三重态能量。缺乏氧取代基的吲哚的 2,3-二氧杂环丁烷的分解产物具有比 NFX 更高的三重态能量,并且能够光敏 CPD 的形成,这表明色氨酸的过氧亚硝酸盐氧化可能在 CPD 的黑暗途径中发挥迄今为止未被认识的作用。黑色素前体 DHI 和 DHICA 的 3-二氧杂环丁烷将具有类似的低三重态能量。缺乏氧取代基的吲哚的 2,3-二氧杂环丁烷的分解产物具有比 NFX 更高的三重态能量,并且能够光敏 CPD 的形成,这表明色氨酸的过氧亚硝酸盐氧化可能在 CPD 的黑暗途径中发挥迄今为止未被认识的作用。黑色素前体 DHI 和 DHICA 的 3-二氧杂环丁烷将具有类似的低三重态能量。缺乏氧取代基的吲哚的 2,3-二氧杂环丁烷的分解产物具有比 NFX 更高的三重态能量,并且能够光敏 CPD 的形成,这表明色氨酸的过氧亚硝酸盐氧化可能在 CPD 的黑暗途径中发挥迄今为止未被认识的作用。
更新日期:2021-09-24
中文翻译:

吲哚的 2,3-二氧杂环丁烷的假定分解产物对环丁烷嘧啶二聚体 (CPD) 形成的光敏能力及其对黑素细胞中 CPD 的“暗”(化学致敏)途径的影响†
通过黑色素细胞中的“暗”途径形成环丁烷嘧啶二聚体 (CPD) 归因于黑色素或黑色素前体的过氧亚硝酸盐氧化产生的二氧杂环丁烷的化学敏化作用。建议这些二氧杂环丁烷分解成三重态化合物,通过三重态-三重态能量转移使 CPD 形成敏感。为了确定这些化合物是否能够敏化 CPD 的形成,合成了各种取代的吲哚的 2,3-二氧杂环丁烷的假定分解产物,并在 77 K 下测定了它们的三重态能量。它们对 CPD 形成的光敏化能力由酶确定。与具有最低三重态能量的诺氟沙星 (NFX) 相比,偶联凝胶电泳分析对 CPD 形成敏感。2的分解产物,用作黑色素前体模型的 5-羟基和 5,6-二甲氧基吲哚的 3-二氧杂环丁烷具有较低的三重态能量并且不能形成光敏 CPD。理论计算表明,黑色素前体 DHI 和 DHICA 的 2,3-二氧杂环丁烷的分解产物将具有类似的低三重态能量。缺乏氧取代基的吲哚的 2,3-二氧杂环丁烷的分解产物具有比 NFX 更高的三重态能量,并且能够光敏 CPD 的形成,这表明色氨酸的过氧亚硝酸盐氧化可能在 CPD 的黑暗途径中发挥迄今为止未被认识的作用。黑色素前体 DHI 和 DHICA 的 3-二氧杂环丁烷将具有类似的低三重态能量。缺乏氧取代基的吲哚的 2,3-二氧杂环丁烷的分解产物具有比 NFX 更高的三重态能量,并且能够光敏 CPD 的形成,这表明色氨酸的过氧亚硝酸盐氧化可能在 CPD 的黑暗途径中发挥迄今为止未被认识的作用。黑色素前体 DHI 和 DHICA 的 3-二氧杂环丁烷将具有类似的低三重态能量。缺乏氧取代基的吲哚的 2,3-二氧杂环丁烷的分解产物具有比 NFX 更高的三重态能量,并且能够光敏 CPD 的形成,这表明色氨酸的过氧亚硝酸盐氧化可能在 CPD 的黑暗途径中发挥迄今为止未被认识的作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号