当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Z-Selective Synthesis of α-Sulfanyl Carbonyl Compounds from Internal Alkynes and Thiols via Photoredox Catalysis
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2021-09-23 , DOI: 10.1002/adsc.202100996 Alberto Luridiana 1 , Angelo Frongia 2 , Mariano Scorciapino 3 , Giuliano Malloci 3 , Barbara Manconi 4 , Simone Serrao 3 , Pier Carlo Ricci 3 , Francesco Secci 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2021-09-23 , DOI: 10.1002/adsc.202100996 Alberto Luridiana 1 , Angelo Frongia 2 , Mariano Scorciapino 3 , Giuliano Malloci 3 , Barbara Manconi 4 , Simone Serrao 3 , Pier Carlo Ricci 3 , Francesco Secci 1
Affiliation
|
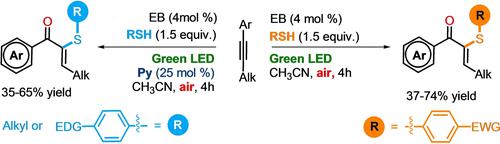
A synthetically useful Z-selective cascade formal thiyl radical addition, 1,3-double bond isomerization, oxygen trapping reaction, can be promoted by Eosin B under visible light, leading to the construction of 2-aryl- and alkylthio enone derivatives in good yields. An accurate study on the reactivity of different thiols and the screening of the reaction conditions, allowed us to extend this reaction to a large number of substrates, showing a good functional groups tolerance while highlighting the limitations of this procedure. Background experiments and mechanistic studies were also. performed to rationalize this cascade process. The usefulness of this methodology was finally demonstrated via the transformation of a series of α-sulfanyl-enone adducts through selected oxidation reactions, stereoselective synthesis of cyclopropyl ketones, indanones, and pyrazole compounds.
中文翻译:

通过光氧化还原催化从内炔烃和硫醇 Z 选择性合成 α-磺基羰基化合物
综合有用的Z-选择性级联甲硫基自由基加成、1,3-双键异构化、氧捕获反应,可以在可见光下被曙红B促进,从而以良好的产率构建2-芳基-和烷硫基烯酮衍生物。对不同硫醇的反应性和反应条件筛选的准确研究,使我们能够将该反应扩展到大量底物,显示出良好的官能团耐受性,同时突出了该过程的局限性。背景实验和机理研究也是如此。执行以合理化此级联过程。通过选择性氧化反应、环丙基酮、茚满酮和吡唑化合物的立体选择性合成转化一系列 α-硫烷基-烯酮加合物,最终证明了该方法的有效性。
更新日期:2021-09-23
中文翻译:

通过光氧化还原催化从内炔烃和硫醇 Z 选择性合成 α-磺基羰基化合物
综合有用的Z-选择性级联甲硫基自由基加成、1,3-双键异构化、氧捕获反应,可以在可见光下被曙红B促进,从而以良好的产率构建2-芳基-和烷硫基烯酮衍生物。对不同硫醇的反应性和反应条件筛选的准确研究,使我们能够将该反应扩展到大量底物,显示出良好的官能团耐受性,同时突出了该过程的局限性。背景实验和机理研究也是如此。执行以合理化此级联过程。通过选择性氧化反应、环丙基酮、茚满酮和吡唑化合物的立体选择性合成转化一系列 α-硫烷基-烯酮加合物,最终证明了该方法的有效性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号