当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Exploiting Configurational Lability in Aza-Sulfur Compounds for the Organocatalytic Enantioselective Synthesis of Sulfonimidamides
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2021-09-24 , DOI: 10.1002/anie.202109160 Michael J Tilby 1 , Damien F Dewez 1 , Adrian Hall 2 , Carolina Martínez Lamenca 3 , Michael C Willis 1
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2021-09-24 , DOI: 10.1002/anie.202109160 Michael J Tilby 1 , Damien F Dewez 1 , Adrian Hall 2 , Carolina Martínez Lamenca 3 , Michael C Willis 1
Affiliation
|
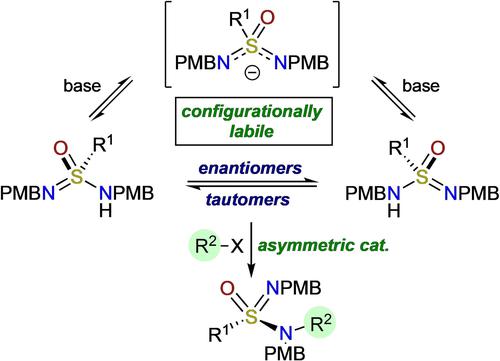
The unusual stereochemical feature of certain sulfonimidamides, in which tautomeric forms are also enantiomeric, has been exploited to develop a catalytic enantioselective synthesis of alkylated sulfonimidamides. The reactions proceed through a prochiral anionic intermediate to deliver enantiomerically enriched products. A double-alkylated, cinchona alkaloid-derived catalyst is used as a phase-transfer catalyst.

中文翻译:

利用氮杂硫化合物的构型不稳定性用于磺酰亚胺的有机催化对映选择性合成
某些磺酰亚胺的不寻常立体化学特征(其中互变异构形式也是对映异构的)已被用于开发烷基化磺酰亚胺的催化对映选择性合成。反应通过前手性阴离子中间体进行,以提供对映体富集的产物。双烷基化的金鸡纳生物碱衍生催化剂用作相转移催化剂。

更新日期:2021-11-24

中文翻译:

利用氮杂硫化合物的构型不稳定性用于磺酰亚胺的有机催化对映选择性合成
某些磺酰亚胺的不寻常立体化学特征(其中互变异构形式也是对映异构的)已被用于开发烷基化磺酰亚胺的催化对映选择性合成。反应通过前手性阴离子中间体进行,以提供对映体富集的产物。双烷基化的金鸡纳生物碱衍生催化剂用作相转移催化剂。




























 京公网安备 11010802027423号
京公网安备 11010802027423号