当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
gem-Difluoroallylation of Aryl Halides and Pseudo Halides with Difluoroallylboron Reagents in High Regioselectivity
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2021-09-23 , DOI: 10.1002/anie.202111476 Shu Sakamoto 1, 2 , Trevor W Butcher 1 , Jonathan L Yang 1 , John F Hartwig 1
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2021-09-23 , DOI: 10.1002/anie.202111476 Shu Sakamoto 1, 2 , Trevor W Butcher 1 , Jonathan L Yang 1 , John F Hartwig 1
Affiliation
|
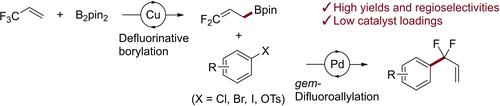
The coupling of a difluoroallylboronate with aryl and heteroaryl halides and triflates provides a convenient and broadly applicable synthesis of difluoroallylarenes. The difluoroallyl boron reagent is formed by a copper-catalyzed defluorinative borylation of the inexpensive reagent 3,3,3-trifluoropropene, and the products undergo a wide range of reactions to a series of difluoro-substituted analogs of common biologically valuable building blocks.

中文翻译:

高区域选择性二氟烯丙基硼试剂对芳基卤化物和拟卤化物进行偕二氟烯丙基化
二氟烯丙基硼酸酯与芳基和杂芳基卤化物和三氟甲磺酸酯的偶联提供了方便且广泛适用的二氟烯丙基芳烃的合成。二氟烯丙基硼试剂是通过铜催化的廉价试剂 3,3,3-三氟丙烯的脱氟硼基化形成的,并且产物经历广泛的反应,形成一系列常见的具有生物价值的结构单元的二氟取代的类似物。

更新日期:2021-11-24

中文翻译:

高区域选择性二氟烯丙基硼试剂对芳基卤化物和拟卤化物进行偕二氟烯丙基化
二氟烯丙基硼酸酯与芳基和杂芳基卤化物和三氟甲磺酸酯的偶联提供了方便且广泛适用的二氟烯丙基芳烃的合成。二氟烯丙基硼试剂是通过铜催化的廉价试剂 3,3,3-三氟丙烯的脱氟硼基化形成的,并且产物经历广泛的反应,形成一系列常见的具有生物价值的结构单元的二氟取代的类似物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号