Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Activatable Biomineralized Nanoplatform Remodels the Intracellular Environment of Multidrug-Resistant Tumors for Enhanced Ferroptosis/Apoptosis Therapy
Small ( IF 13.3 ) Pub Date : 2021-09-23 , DOI: 10.1002/smll.202102269 Xuan Wang 1 , Yuanyuan Zhao 1 , Yan Hu 2 , Yang Fei 1 , Youbo Zhao 1 , Chencheng Xue 1 , Kaiyong Cai 2 , Menghuan Li 1 , Zhong Luo 1
Small ( IF 13.3 ) Pub Date : 2021-09-23 , DOI: 10.1002/smll.202102269 Xuan Wang 1 , Yuanyuan Zhao 1 , Yan Hu 2 , Yang Fei 1 , Youbo Zhao 1 , Chencheng Xue 1 , Kaiyong Cai 2 , Menghuan Li 1 , Zhong Luo 1
Affiliation
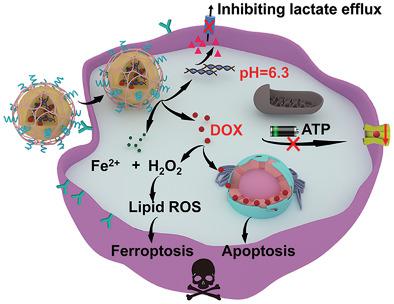
|
Ferroptosis is a new form of regulated cell death with significant therapeutic prospect, but its application against drug-resistant tumor cells is challenging due to their ability to effuse antitumor agents via p-glycoprotein (P-gp) and anti-lipid peroxidation alkaline intracellular environment. Herein, an amorphous calcium phosphate (ACP)-based nanoplatform is reported for the targeted combinational ferroptosis/apoptosis therapy of drug resistant tumor cells by blocking the MCT4-mediated efflux of lactic acid (LA). The nanoplatform is fabricated through the biomineralization of doxorubicin-Fe2+ (DOX-Fe2+) complex and MCT4-inhibiting siRNAs (siMCT4) and can release them to the tumor cytoplasm after the hydrolysis of ACP and dissociation of DOX-Fe2+ in the acidic lysosomes. siMCT4 can inhibit MCT4 expression and force the glycolysis-generated lactic acid (LA) to remain in cytoplasm for rapid acidification. The nanoplatform-induced remodeling of the tumor intracellular environment can not only interrupt the ATP supply required for P-gp-dependent DOX effusion to enhance H2O2 production, but also increase the overall catalytic efficiency of Fe2+ for the initiation and propagation of lipid peroxidation. These features could act in concert to enhance the efficacy of the combinational ferroptosis/chemotherapy and prolong the survival of tumor-bearing mice. This study may provide new avenues for the treatment of multidrug-resistant tumors.
中文翻译:

可激活的生物矿化纳米平台重塑多药耐药肿瘤的细胞内环境,用于增强铁死亡/细胞凋亡治疗
Ferroptosis 是一种具有重要治疗前景的受调节细胞死亡的新形式,但由于它们能够通过 p-糖蛋白 (P-gp) 和抗脂质过氧化碱性细胞内环境释放抗肿瘤药物,因此其对耐药肿瘤细胞的应用具有挑战性. 在此,报道了一种基于无定形磷酸钙 (ACP) 的纳米平台,通过阻断 MCT4 介导的乳酸 (LA) 外流,对耐药肿瘤细胞进行靶向联合铁死亡/凋亡治疗。该纳米平台是通过阿霉素-Fe 2+ (DOX-Fe 2+ ) 复合物和 MCT4 抑制 siRNA (siMCT4)的生物矿化制成的,并且可以在 ACP 水解和 DOX-Fe 2+解离后将它们释放到肿瘤细胞质中在酸性溶酶体中。siMCT4 可以抑制 MCT4 表达并迫使糖酵解产生的乳酸 (LA) 留在细胞质中以进行快速酸化。纳米平台诱导的肿瘤细胞内环境重塑不仅可以中断 P-gp 依赖性 DOX 渗出所需的 ATP 供应,以提高 H 2 O 2 的产生,而且还可以提高 Fe 2+的整体催化效率,以启动和传播脂质过氧化。这些特征可以协同作用,提高铁死亡/化疗联合治疗的疗效,并延长荷瘤小鼠的生存期。该研究可能为多药耐药肿瘤的治疗提供新的途径。
更新日期:2021-11-25
中文翻译:

可激活的生物矿化纳米平台重塑多药耐药肿瘤的细胞内环境,用于增强铁死亡/细胞凋亡治疗
Ferroptosis 是一种具有重要治疗前景的受调节细胞死亡的新形式,但由于它们能够通过 p-糖蛋白 (P-gp) 和抗脂质过氧化碱性细胞内环境释放抗肿瘤药物,因此其对耐药肿瘤细胞的应用具有挑战性. 在此,报道了一种基于无定形磷酸钙 (ACP) 的纳米平台,通过阻断 MCT4 介导的乳酸 (LA) 外流,对耐药肿瘤细胞进行靶向联合铁死亡/凋亡治疗。该纳米平台是通过阿霉素-Fe 2+ (DOX-Fe 2+ ) 复合物和 MCT4 抑制 siRNA (siMCT4)的生物矿化制成的,并且可以在 ACP 水解和 DOX-Fe 2+解离后将它们释放到肿瘤细胞质中在酸性溶酶体中。siMCT4 可以抑制 MCT4 表达并迫使糖酵解产生的乳酸 (LA) 留在细胞质中以进行快速酸化。纳米平台诱导的肿瘤细胞内环境重塑不仅可以中断 P-gp 依赖性 DOX 渗出所需的 ATP 供应,以提高 H 2 O 2 的产生,而且还可以提高 Fe 2+的整体催化效率,以启动和传播脂质过氧化。这些特征可以协同作用,提高铁死亡/化疗联合治疗的疗效,并延长荷瘤小鼠的生存期。该研究可能为多药耐药肿瘤的治疗提供新的途径。


























 京公网安备 11010802027423号
京公网安备 11010802027423号