当前位置:
X-MOL 学术
›
J. Phys. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Hydride transfer versus H-abstraction in the reaction of O3 with 2-propanol: The influence of solvent
Journal of Physical Organic Chemistry ( IF 1.8 ) Pub Date : 2021-09-22 , DOI: 10.1002/poc.4288 Erika Reisz 1 , Sergej Naumov 2 , Agnes Tekle‐Röttering 3 , Winfried Schmidt 3
Journal of Physical Organic Chemistry ( IF 1.8 ) Pub Date : 2021-09-22 , DOI: 10.1002/poc.4288 Erika Reisz 1 , Sergej Naumov 2 , Agnes Tekle‐Röttering 3 , Winfried Schmidt 3
Affiliation
|
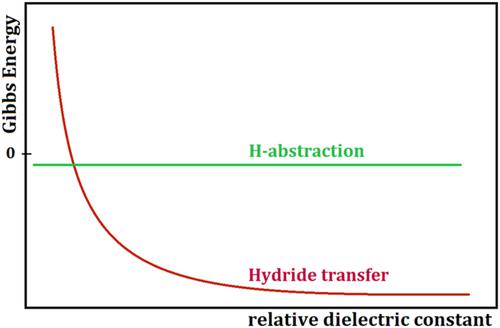
The main purpose of this paper was to systematically investigate the influence of the solvent on mechanism behind O3 reaction with 2-propanol. Gibbs energies were calculated for the reactions that initiated the possible mechanisms: hydride transfer ((H3C)2(HO)C–H(solv) + O3(solv) → (H3C)2(HO)C+(solv) + HO3−(solv)) and H-abstraction ((H3C)2(HO)C–H(solv) + O3(solv) → (H3C)2(HO)C•(solv) + HO3•(solv)) under working conditions with various solvents. The characteristic of the solvent used for calculations was the relative dielectric constant, ɛ, which ranged from 2.02 (cyclohexane) to 80 (water). The calculation method was based on density functional theory (DFT). In addition, this study took into consideration the conversion of the radical pair as products of H-abstraction to the ion pair formed following hydride transfer; the conversion occurred by electron transfer ((H3C)2(HO)C•(solv) + HO3•(solv) → (H3C)2(HO)C+(solv) + HO3−(solv)). Our calculations showed that the reaction of O3 with 2-propanol was triggered by H-abstraction in nonpolar solvents, but in polar solvents, mainly by a pseudo-hydride transfer consisting of H-abstraction followed by electron transfer between the radicals formed by H-abstraction. The transition state formed en route to products had a linear structure for water, whereas for cyclohexane, linear and cyclic transition states coexisted.
中文翻译:

O3 与 2-丙醇反应中的氢化物转移与 H-提取:溶剂的影响
本文的主要目的是系统研究溶剂对O 3与2-丙醇反应机理的影响。计算引发可能机制的反应的吉布斯能量:氢化物转移 ((H 3 C) 2 (HO)C–H (solv) + O 3(solv) → (H 3 C) 2 (HO)C + ( solv) + HO 3 - (solv) ) 和 H-抽象 ((H 3 C) 2 (HO)C–H (solv) + O 3(solv) → (H 3 C) 2 (HO)C• (solv ) + 水3 • (solv) ) 在各种溶剂的工作条件下。用于计算的溶剂的特性是相对介电常数 ɛ,范围从 2.02(环己烷)到 80(水)。计算方法基于密度泛函理论(DFT)。此外,本研究还考虑了自由基对作为 H 抽象产物的转化为氢化物转移后形成的离子对;通过电子转移发生转化 ((H 3 C) 2 (HO)C• (solv) + HO 3 • (solv) → (H 3 C) 2 (HO)C + (solv) + HO 3 -(解决))。我们的计算表明,O 3与 2-丙醇的反应是由非极性溶剂中的 H 提取引发的,但在极性溶剂中,主要是由 H 提取组成的拟氢化物转移,然后是 H 形成的自由基之间的电子转移。 -抽象。在生成产物的过程中形成的过渡态对于水具有线性结构,而对于环己烷,线性和环状过渡态并存。
更新日期:2021-09-22
中文翻译:

O3 与 2-丙醇反应中的氢化物转移与 H-提取:溶剂的影响
本文的主要目的是系统研究溶剂对O 3与2-丙醇反应机理的影响。计算引发可能机制的反应的吉布斯能量:氢化物转移 ((H 3 C) 2 (HO)C–H (solv) + O 3(solv) → (H 3 C) 2 (HO)C + ( solv) + HO 3 - (solv) ) 和 H-抽象 ((H 3 C) 2 (HO)C–H (solv) + O 3(solv) → (H 3 C) 2 (HO)C• (solv ) + 水3 • (solv) ) 在各种溶剂的工作条件下。用于计算的溶剂的特性是相对介电常数 ɛ,范围从 2.02(环己烷)到 80(水)。计算方法基于密度泛函理论(DFT)。此外,本研究还考虑了自由基对作为 H 抽象产物的转化为氢化物转移后形成的离子对;通过电子转移发生转化 ((H 3 C) 2 (HO)C• (solv) + HO 3 • (solv) → (H 3 C) 2 (HO)C + (solv) + HO 3 -(解决))。我们的计算表明,O 3与 2-丙醇的反应是由非极性溶剂中的 H 提取引发的,但在极性溶剂中,主要是由 H 提取组成的拟氢化物转移,然后是 H 形成的自由基之间的电子转移。 -抽象。在生成产物的过程中形成的过渡态对于水具有线性结构,而对于环己烷,线性和环状过渡态并存。


























 京公网安备 11010802027423号
京公网安备 11010802027423号