当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Deorphanizing Caspase-3 and Caspase-9 Substrates In and Out of Apoptosis with Deep Substrate Profiling
ACS Chemical Biology ( IF 4 ) Pub Date : 2021-09-23 , DOI: 10.1021/acschembio.1c00456 Luam E Araya 1 , Ishankumar V Soni 2 , Jeanne A Hardy 2 , Olivier Julien 1
ACS Chemical Biology ( IF 4 ) Pub Date : 2021-09-23 , DOI: 10.1021/acschembio.1c00456 Luam E Araya 1 , Ishankumar V Soni 2 , Jeanne A Hardy 2 , Olivier Julien 1
Affiliation
|
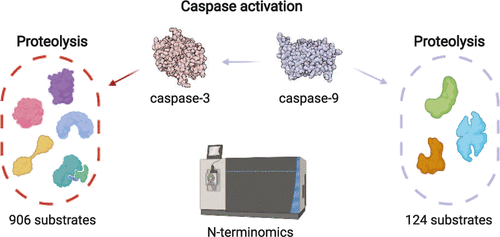
Caspases are a family of enzymes that regulate biological processes such as inflammation and programmed cell death, through proteolysis. For example, in the intrinsic pathway of apoptosis, cell death signaling involves cytochrome c release from the mitochondria, which leads to the activation of caspase-9 and eventually the executioners caspase-3 and -7. One key step in our understanding of these proteases is to identify their respective protein substrates. Although hundreds of substrates have been linked to caspase-3, only a small handful of substrates have been reported for caspase-9. Employing deep profiling by subtiligase N-terminomics, we present here an unbiased analysis of caspase-3 and caspase-9 substrates in native cell lysates. We identified 906 putative protein substrates associated with caspase-3 and 124 protein substrates for caspase-9. This is the most comprehensive list of caspase substrates reported for each of these proteases, revealing a pool of new substrates that could not have been discovered using other approaches. Over half of the caspase-9 substrates were also cleaved by caspase-3, but often at unique sites, suggesting an evolved functional redundancy for these two proteases. Correspondingly, nearly half of the caspase-9 cleavage sites were not recognized by caspase-3. Our results suggest that in addition to its important role in activating the executioners, the role of caspase-9 is likely broader and more complex than previously appreciated, which includes proteolysis of key apoptotic substrates other than just caspase-3 and -7 and involvement in non-apoptotic pathways. Our results are well poised to aid the discovery of new biological functions for these two caspases.
中文翻译:

用深度底物分析使 Caspase-3 和 Caspase-9 底物进出细胞凋亡
半胱天冬酶是一个酶家族,通过蛋白水解调节炎症和程序性细胞死亡等生物过程。例如,在细胞凋亡的内在途径中,细胞死亡信号传导涉及细胞色素c从线粒体释放,这导致 caspase-9 的激活,最终导致刽子手 caspase-3 和 -7 的激活。我们了解这些蛋白酶的一个关键步骤是识别它们各自的蛋白质底物。尽管已有数百种底物与 caspase-3 相关联,但据报道只有少数底物与 caspase-9 相关。通过 subtiligase N-terminomics 进行深度分析,我们在这里对天然细胞裂解物中的 caspase-3 和 caspase-9 底物进行了公正的分析。我们确定了 906 个与 caspase-3 相关的推定蛋白质底物和 124 个与 caspase-9 相关的蛋白质底物。这是针对每种蛋白酶报告的最全面的半胱天冬酶底物列表,揭示了使用其他方法无法发现的新底物库。超过一半的 caspase-9 底物也被 caspase-3 切割,但通常在独特的位点,这表明这两种蛋白酶存在进化的功能冗余。相应地,近一半的 caspase-9 切割位点未被 caspase-3 识别。我们的研究结果表明,除了在激活刽子手方面的重要作用外,caspase-9 的作用可能比以前认识到的更广泛和更复杂,其中包括除 caspase-3 和 -7 之外的关键凋亡底物的蛋白水解以及参与非凋亡途径。我们的结果很好地有助于发现这两种半胱天冬酶的新生物学功能。近一半的 caspase-9 切割位点未被 caspase-3 识别。我们的研究结果表明,除了在激活刽子手方面的重要作用外,caspase-9 的作用可能比以前认识到的更广泛和更复杂,其中包括除 caspase-3 和 -7 之外的关键凋亡底物的蛋白水解以及参与非凋亡途径。我们的结果很好地有助于发现这两种半胱天冬酶的新生物学功能。近一半的 caspase-9 切割位点未被 caspase-3 识别。我们的研究结果表明,除了在激活刽子手方面的重要作用外,caspase-9 的作用可能比以前认识到的更广泛和更复杂,其中包括除 caspase-3 和 -7 之外的关键凋亡底物的蛋白水解以及参与非凋亡途径。我们的结果很好地有助于发现这两种半胱天冬酶的新生物学功能。
更新日期:2021-11-19
中文翻译:

用深度底物分析使 Caspase-3 和 Caspase-9 底物进出细胞凋亡
半胱天冬酶是一个酶家族,通过蛋白水解调节炎症和程序性细胞死亡等生物过程。例如,在细胞凋亡的内在途径中,细胞死亡信号传导涉及细胞色素c从线粒体释放,这导致 caspase-9 的激活,最终导致刽子手 caspase-3 和 -7 的激活。我们了解这些蛋白酶的一个关键步骤是识别它们各自的蛋白质底物。尽管已有数百种底物与 caspase-3 相关联,但据报道只有少数底物与 caspase-9 相关。通过 subtiligase N-terminomics 进行深度分析,我们在这里对天然细胞裂解物中的 caspase-3 和 caspase-9 底物进行了公正的分析。我们确定了 906 个与 caspase-3 相关的推定蛋白质底物和 124 个与 caspase-9 相关的蛋白质底物。这是针对每种蛋白酶报告的最全面的半胱天冬酶底物列表,揭示了使用其他方法无法发现的新底物库。超过一半的 caspase-9 底物也被 caspase-3 切割,但通常在独特的位点,这表明这两种蛋白酶存在进化的功能冗余。相应地,近一半的 caspase-9 切割位点未被 caspase-3 识别。我们的研究结果表明,除了在激活刽子手方面的重要作用外,caspase-9 的作用可能比以前认识到的更广泛和更复杂,其中包括除 caspase-3 和 -7 之外的关键凋亡底物的蛋白水解以及参与非凋亡途径。我们的结果很好地有助于发现这两种半胱天冬酶的新生物学功能。近一半的 caspase-9 切割位点未被 caspase-3 识别。我们的研究结果表明,除了在激活刽子手方面的重要作用外,caspase-9 的作用可能比以前认识到的更广泛和更复杂,其中包括除 caspase-3 和 -7 之外的关键凋亡底物的蛋白水解以及参与非凋亡途径。我们的结果很好地有助于发现这两种半胱天冬酶的新生物学功能。近一半的 caspase-9 切割位点未被 caspase-3 识别。我们的研究结果表明,除了在激活刽子手方面的重要作用外,caspase-9 的作用可能比以前认识到的更广泛和更复杂,其中包括除 caspase-3 和 -7 之外的关键凋亡底物的蛋白水解以及参与非凋亡途径。我们的结果很好地有助于发现这两种半胱天冬酶的新生物学功能。



























 京公网安备 11010802027423号
京公网安备 11010802027423号