当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Selective Chloride-Mediated Neat Ethanol Oxidation to 1,1-Diethoxyethane via an Electrochemically Generated Ethyl Hypochlorite Intermediate
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2021-09-23 , DOI: 10.1021/jacs.1c05976 Siqi Li 1 , Bart M Bartlett 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2021-09-23 , DOI: 10.1021/jacs.1c05976 Siqi Li 1 , Bart M Bartlett 1
Affiliation
|
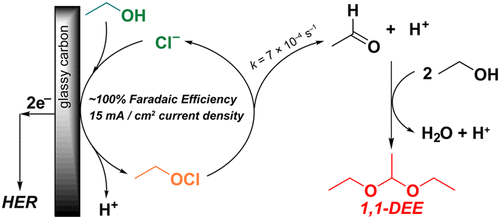
Selective primary alcohol oxidation to form aldehydes products without overoxidation to carboxylic acids remains a key chemistry challenge. Using simple alkylammonium chloride as the electrolyte with a glassy carbon working electrode in neat ethanol solvent, 1,1-diethoxyethane (DEE) was prepared with >95% faradaic efficiency (FE). DEE serves as a storage platform protecting acetaldehyde from overoxidation and volatilization. UV–vis spectroscopy shows that the reaction proceeds through an ethyl hypochlorite intermediate as the sole chloride oxidation product, and that this intermediate decomposes unimolecularly (rate constant k = (6.896 ± 0.516) × 10–4 s–1) to form HCl catalyst and acetaldehyde, which undergoes rapid nucleophilic attack by ethanol solvent to form the DEE product. This indirect oxidation mechanism enables ethanol oxidation at much less positive potentials due to the fast kinetics for chloride anion oxidation.
中文翻译:

通过电化学生成的次氯酸乙酯中间体选择性氯化物介导的纯乙醇氧化为 1,1-二乙氧基乙烷
选择性伯醇氧化形成醛类产品而不过度氧化成羧酸仍然是一个关键的化学挑战。使用简单的烷基氯化铵作为电解质,在纯乙醇溶剂中使用玻璃碳工作电极,以 > 95% 的法拉第效率 (FE) 制备 1,1-二乙氧基乙烷 (DEE)。DEE 用作保护乙醛免受过度氧化和挥发的储存平台。紫外-可见分光光度法显示反应通过次氯酸乙酯中间体作为唯一的氯化物氧化产物进行,并且该中间体以单分子方式分解(速率常数k = (6.896 ± 0.516) × 10 –4 s –1) 形成 HCl 催化剂和乙醛,乙醛经乙醇溶剂快速亲核攻击,形成 DEE 产物。由于氯阴离子氧化的快速动力学,这种间接氧化机制能够在低得多的正电位下进行乙醇氧化。
更新日期:2021-10-06
中文翻译:

通过电化学生成的次氯酸乙酯中间体选择性氯化物介导的纯乙醇氧化为 1,1-二乙氧基乙烷
选择性伯醇氧化形成醛类产品而不过度氧化成羧酸仍然是一个关键的化学挑战。使用简单的烷基氯化铵作为电解质,在纯乙醇溶剂中使用玻璃碳工作电极,以 > 95% 的法拉第效率 (FE) 制备 1,1-二乙氧基乙烷 (DEE)。DEE 用作保护乙醛免受过度氧化和挥发的储存平台。紫外-可见分光光度法显示反应通过次氯酸乙酯中间体作为唯一的氯化物氧化产物进行,并且该中间体以单分子方式分解(速率常数k = (6.896 ± 0.516) × 10 –4 s –1) 形成 HCl 催化剂和乙醛,乙醛经乙醇溶剂快速亲核攻击,形成 DEE 产物。由于氯阴离子氧化的快速动力学,这种间接氧化机制能够在低得多的正电位下进行乙醇氧化。


























 京公网安备 11010802027423号
京公网安备 11010802027423号