Acta Pharmaceutica Sinica B ( IF 14.5 ) Pub Date : 2021-09-22 , DOI: 10.1016/j.apsb.2021.09.014 Min-Xia Su 1 , Yu-Lian Xu 1 , Xiao-Ming Jiang 1 , Mu-Yang Huang 1 , Le-Le Zhang 1 , Luo-Wei Yuan 1 , Xiao-Huang Xu 1 , Qi Zhu 1 , Jian-Li Gao 2 , Jia-Hong Lu 1 , Xiuping Chen 1 , Ming-Qing Huang 3 , Yitao Wang 1 , Jin-Jian Lu 1, 4
|
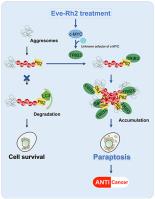
The mammalian target of rapamycin (mTOR) pathway is abnormally activated in lung cancer. However, the anti-lung cancer effect of mTOR inhibitors as monotherapy is modest. Here, we identified that ginsenoside Rh2, an active component of Panax ginseng C. A. Mey., enhanced the anti-cancer effect of the mTOR inhibitor everolimus both in vitro and in vivo. Moreover, ginsenoside Rh2 alleviated the hepatic fat accumulation caused by everolimus in xenograft nude mice models. The combination of everolimus and ginsenoside Rh2 (labeled Eve-Rh2) induced caspase-independent cell death and cytoplasmic vacuolation in lung cancer cells, indicating that Eve-Rh2 prevented tumor progression by triggering paraptosis. Eve-Rh2 up-regulated the expression of c-MYC in cancer cells as well as tumor tissues. The increased c-MYC mediated the accumulation of tribbles homolog 3 (TRIB3)/P62+ aggresomes and consequently triggered paraptosis, bypassing the classical c-MYC/MAX pathway. Our study offers a potential effective and safe strategy for the treatment of lung cancer. Moreover, we have identified a new mechanism of TRIB3/P62+ aggresomes-triggered paraptosis and revealed a unique function of c-MYC.
中文翻译:

c-MYC 介导的 TRIB3/P62+ 聚集体积累在依维莫司和人参皂甙 Rh2 联合用药时触发下垂
哺乳动物雷帕霉素靶蛋白 (mTOR) 通路在肺癌中异常激活。然而,mTOR抑制剂作为单一疗法的抗肺癌作用是适度的。在这里,我们发现人参皂甙 Rh2(人参CA Mey. 的活性成分)增强了 mTOR 抑制剂依维莫司在体外和体内的抗癌作用. 此外,人参皂甙Rh2减轻了异种移植裸鼠模型中依维莫司引起的肝脏脂肪堆积。依维莫司和人参皂甙Rh2(标记为Eve-Rh2)的组合在肺癌细胞中诱导不依赖半胱天冬酶的细胞死亡和细胞质空泡化,表明Eve-Rh2通过触发截瘫来阻止肿瘤进展。Eve-Rh2 上调癌细胞和肿瘤组织中 c-MYC 的表达。增加的 c-MYC 介导了 tribbles 同源物 3 (TRIB3)/P62 +聚集体的积累,从而引发了截肢,绕过了经典的 c-MYC/MAX 途径。我们的研究为肺癌的治疗提供了一种潜在的有效和安全的策略。此外,我们确定了TRIB3/P62 +的新机制聚集体触发的下垂并揭示了 c-MYC 的独特功能。


























 京公网安备 11010802027423号
京公网安备 11010802027423号