Water Research ( IF 12.8 ) Pub Date : 2021-09-22 , DOI: 10.1016/j.watres.2021.117676 Zhongfei Ren 1 , Ulrich Bergmann 2 , Tiina Leiviskä 1
|
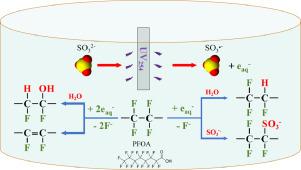
Hydrated electrons (e-aq, E= -2.9 V) generated by advanced reduction processes (ARPs) have been proved to be a promising approach to eliminate various per- and polyfluoroalkyl substances (PFASs) in water. In this study, the decomposition of perfluorooctanoic acid (PFOA) in a complex water matrix by e-aq generated from the UV/sulfite process was investigated. The effect of pH (9–12) and co-existing compounds (chloride, nitrate, phosphate, carbonate and humic acid) on PFOA degradation efficiency was studied. In addition, the intermediates and possible degradation pathways were analyzed by ultra-high performance liquid chromatography-tandem mass spectrometry (UPLC-MS). The results showed that the concentration of PFOA was below the detection limit (10 μg/L) after 1 h (conditions: C0 10 mg/L, initial pH = 10, sulfite 10 mM) while 89% defluorination was achieved after 24 h. Using a higher initial pH (pH = 12) greatly enhanced the PFOA degradation as 100% degradation and 98% defluorination were achieved after 24 h. The presence of carbonate (> 5 mM), nitrate (> 2 mM) and humic acid (> 25 mg/L) showed a significant negative effect on PFOA degradation via a UV blocking effect or quenching of hydrated electrons while the presence of chloride and phosphate had a smaller effect on PFOA degradation. Even at extremely high concentrations of chloride (1.709 M, pH = 11.25), the defluorination ratio reached 97% after 24 h of reaction time. During the process, short-chain perfluorinated carboxylic acids (PFCAs, C < 7) and hydrogen substituted compounds were detected, which implies that chain-shortening and H/F change reactions had occurred. Moreover, this confirmed the generation of sulfonated and unsaturated intermediates during the process, which disclosed valuable new mechanistic insights into PFOA degradation.
中文翻译:

使用紫外线/亚硫酸盐工艺还原降解复杂水基质中的全氟辛酸
由高级还原过程 (ARPs) 产生的水合电子 (e - aq, E = -2.9 V) 已被证明是消除水中各种全氟和多氟烷基物质 (PFAS) 的有前途的方法。在这项研究中,全氟辛酸 (PFOA) 在复杂的水基质中通过 e - aq分解研究了紫外线/亚硫酸盐过程中产生的产物。研究了 pH (9-12) 和共存化合物(氯化物、硝酸盐、磷酸盐、碳酸盐和腐殖酸)对全氟辛酸降解效率的影响。此外,通过超高效液相色谱-串联质谱(UPLC-MS)分析了中间体和可能的降解途径。结果表明,经过1 h(条件:C 010 毫克/升,初始 pH = 10,亚硫酸盐 10 mM),而 24 小时后实现了 89% 的脱氟。使用较高的初始 pH 值(pH = 12)大大增强了全氟辛酸降解,因为 24 小时后实现了 100% 降解和 98% 脱氟。碳酸盐 (> 5 mM)、硝酸盐 (> 2 mM) 和腐植酸 (> 25 mg/L) 的存在通过紫外线阻断效应或水合电子的淬灭对 PFOA 降解产生显着的负面影响,而氯化物和磷酸盐对全氟辛酸降解的影响较小。即使在极高浓度的氯化物(1.709 M,pH = 11.25)下,反应时间 24 小时后脱氟率也达到 97%。在此过程中,短链全氟羧酸 (PFCAs, C< 7) 和氢取代的化合物被检测到,这意味着发生了链缩短和 H/F 变化反应。此外,这证实了在该过程中产生了磺化和不饱和中间体,这揭示了对全氟辛酸降解的有价值的新机制见解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号