Redox Biology ( IF 11.4 ) Pub Date : 2021-09-22 , DOI: 10.1016/j.redox.2021.102139 Shengnan Liu 1 , Jingbo Pi 2 , Qiang Zhang 3
|
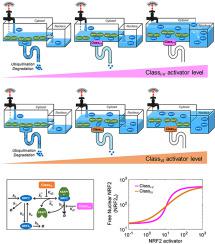
Under oxidative and electrophilic stresses, cells launch an NRF2-mediated transcriptional antioxidant program. The activation of NRF2 depends on a redox sensor, KEAP1, which promotes the ubiquitination and degradation of NRF2. While a great deal has been learned about this duo, its quantitative signaling properties are largely unexplored. Here we examined these properties, including half-life, maximal activation, and response steepness (ultrasensitivity) of NRF2, through mathematical modeling. The models describe the binding of KEAP1 and NRF2 via ETGE and DLG motifs, NRF2 production, KEAP1-dependent and independent NRF2 degradation, and perturbations by different classes of NRF2 activators. Simulations revealed at the basal condition, NRF2 is sequestered by KEAP1 and the KEAP1-NRF2 complex is distributed comparably in an ETGE-bound (open) state and an ETGE and DLG dual-bound (closed) state. When two-step ETGE binding is considered, class I–V, electrophilic NRF2 activators shift the balance to a closed state incompetent to degrade NRF2, while the open and closed KEAP1-NRF2 complexes transition from operating in cycle mode to equilibrium mode. Ultrasensitive NRF2 activation (a steep rise of free NRF2) can occur when NRF2 nearly saturates KEAP1. The ultrasensitivity results from zero-order degradation through DLG binding and protein sequestration through ETGE binding. Optimal abundances of cytosolic and nuclear KEAP1 exist to maximize ultrasensitivity. These response characteristics do not require disruption of DLG binding as suggested by the hinge-latch hypothesis. In comparison, class VI NRF2 activators cause a shift to the open KEAP1-NRF2 complex and ultimately its complete dissociation, resulting in a fast release of NRF2 followed by stabilization. However, ultrasensitivity is lost due to decreasing free KEAP1 abundance. In summary, by simulating the dual role of KEAP1, i.e., sequestering and promoting degradation of NRF2, our modeling provides novel quantitative insights into NRF2 activation, which may help design novel NRF2 modulators and understand the oxidative actions of environmental stressors.
中文翻译:

数学模型揭示了 KEAP1-NRF2 信号的定量特性
在氧化和亲电应激下,细胞启动 NRF2 介导的转录抗氧化程序。NRF2 的激活取决于氧化还原传感器 KEAP1,它促进 NRF2 的泛素化和降解。虽然已经对这对二人组了解了很多,但其定量信号特性在很大程度上尚未得到探索。在这里,我们通过数学建模检查了 NRF2 的这些特性,包括半衰期、最大激活和响应陡度(超敏性)。这些模型描述了 KEAP1 和 NRF2 通过 ETGE 和 DLG 基序的结合、NRF2 的产生、KEAP1 依赖性和独立的 NRF2 降解以及不同类别的 NRF2 激活剂的扰动。在基础条件下显示的模拟,NRF2 被 KEAP1 隔离,并且 KEAP1-NRF2 复合物在 ETGE 结合(开放)状态和 ETGE 和 DLG 双结合(闭合)状态下分布相当。当考虑两步 ETGE 结合时,I-V 类亲电 NRF2 激活剂将平衡转移到无法降解 NRF2 的闭合状态,而开放和闭合的 KEAP1-NRF2 复合物从循环模式转变为平衡模式。当 NRF2 几乎饱和 KEAP1 时,会发生超敏感 NRF2 激活(游离 NRF2 急剧上升)。通过 DLG 结合实现的零级降解和通过 ETGE 结合实现的蛋白质螯合导致了超灵敏性。细胞溶质和核 KEAP1 的最佳丰度存在以最大限度地提高超敏性。这些响应特征不需要像铰链闩锁假设所暗示的那样破坏 DLG 结合。相比下,VI 类 NRF2 激活剂导致向开放的 KEAP1-NRF2 复合物转变并最终完全解离,导致 NRF2 快速释放,随后稳定。然而,由于游离 KEAP1 丰度降低,超敏性丧失。总之,通过模拟 KEAP1 的双重作用,即隔离和促进 NRF2 的降解,我们的模型为 NRF2 激活提供了新的定量见解,这可能有助于设计新的 NRF2 调节剂并了解环境压力源的氧化作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号