当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Metal-Free One-Pot Synthesis of 2-(2-Hydrazinyl) Thiazole Derivatives Using Graphene Oxide in a Green Solvent and Molecular Docking Studies
ChemistrySelect ( IF 2.1 ) Pub Date : 2021-09-22 , DOI: 10.1002/slct.202102642 Arindam Das 1 , Sovan Dey 1 , Sumit Chakraborty 1 , Anup Barman 1 , Ram Naresh Yadav 2 , Rabiul Gazi 3 , Madhurima Jana 3 , Md. Firoj Hossain 1
ChemistrySelect ( IF 2.1 ) Pub Date : 2021-09-22 , DOI: 10.1002/slct.202102642 Arindam Das 1 , Sovan Dey 1 , Sumit Chakraborty 1 , Anup Barman 1 , Ram Naresh Yadav 2 , Rabiul Gazi 3 , Madhurima Jana 3 , Md. Firoj Hossain 1
Affiliation
|
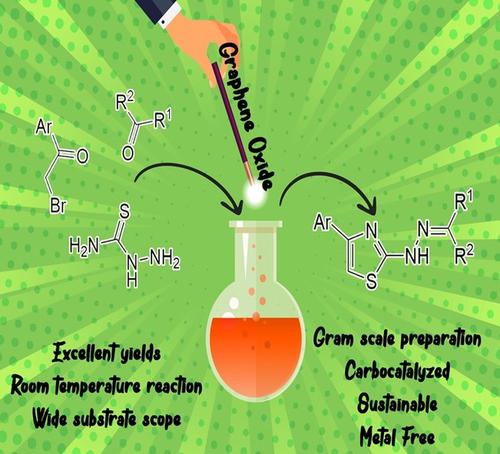
An efficient, one-pot three-component reaction in the synthesis of a wide range of 2,4-disubstituted hydrazinyl thiazole scaffolds in ethanol at room temperature has been developed by the reaction of carbonyl compounds, phenacyl bromides, and thiosemicarbazide, using graphene oxide (GO) as a catalyst. The present catalytic method has many significant advantages such as shorter reaction time, broad substrate scope, low catalyst loading, environmentally benign solvent media, easy handling, and operational simplicity at room temperature. The products are identified based on their various spectroscopic data. The GO catalyst has a high reusability rate and is simple to recover. A mechanistic study suggests that the reaction takes place via a thiosemicarbazone intermediate, which is isolated and confirmed in this study using spectroscopic techniques. Thereafter, a molecular docking study has been performed to evaluate the binding affinity of hydrazinyl thiazole derivative as a possible inhibitor on the protein active site of human α-amylase and glucosamine 6-phosphate synthase
中文翻译:

使用氧化石墨烯在绿色溶剂和分子对接研究中无金属一锅法合成 2-(2-肼基) 噻唑衍生物
通过羰基化合物、苯甲酰溴和氨基硫脲的反应,使用氧化石墨烯,在室温下在乙醇中合成了多种 2,4-二取代肼基噻唑支架的高效、一锅三组分反应(GO) 作为催化剂。该催化方法具有反应时间短、底物范围广、催化剂负载量低、溶剂介质环境友好、易于操作和室温下操作简单等诸多显着优点。产品根据其各种光谱数据进行识别。GO催化剂重复使用率高,回收简单。一项机理研究表明,反应是通过氨基硫脲中间体,在本研究中使用光谱技术分离和确认。此后,进行了一项分子对接研究,以评估肼基噻唑衍生物作为人 α-淀粉酶和葡萄糖胺 6-磷酸合酶蛋白活性位点的可能抑制剂的结合亲和力
更新日期:2021-09-22
中文翻译:

使用氧化石墨烯在绿色溶剂和分子对接研究中无金属一锅法合成 2-(2-肼基) 噻唑衍生物
通过羰基化合物、苯甲酰溴和氨基硫脲的反应,使用氧化石墨烯,在室温下在乙醇中合成了多种 2,4-二取代肼基噻唑支架的高效、一锅三组分反应(GO) 作为催化剂。该催化方法具有反应时间短、底物范围广、催化剂负载量低、溶剂介质环境友好、易于操作和室温下操作简单等诸多显着优点。产品根据其各种光谱数据进行识别。GO催化剂重复使用率高,回收简单。一项机理研究表明,反应是通过氨基硫脲中间体,在本研究中使用光谱技术分离和确认。此后,进行了一项分子对接研究,以评估肼基噻唑衍生物作为人 α-淀粉酶和葡萄糖胺 6-磷酸合酶蛋白活性位点的可能抑制剂的结合亲和力



























 京公网安备 11010802027423号
京公网安备 11010802027423号