当前位置:
X-MOL 学术
›
ChemBioChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Engineering Leifsonia Alcohol Dehydrogenase for Thermostability and Catalytic Efficiency by Enhancing Subunit Interactions
ChemBioChem ( IF 3.2 ) Pub Date : 2021-09-22 , DOI: 10.1002/cbic.202100431 Lu Zhu 1, 2 , Yang Song 3, 4 , Chenchen Chang 3, 4 , Hongmin Ma 2 , Lu Yang 1 , Zixin Deng 1 , Wei Deng 3, 4 , Xudong Qu 1, 2
ChemBioChem ( IF 3.2 ) Pub Date : 2021-09-22 , DOI: 10.1002/cbic.202100431 Lu Zhu 1, 2 , Yang Song 3, 4 , Chenchen Chang 3, 4 , Hongmin Ma 2 , Lu Yang 1 , Zixin Deng 1 , Wei Deng 3, 4 , Xudong Qu 1, 2
Affiliation
|
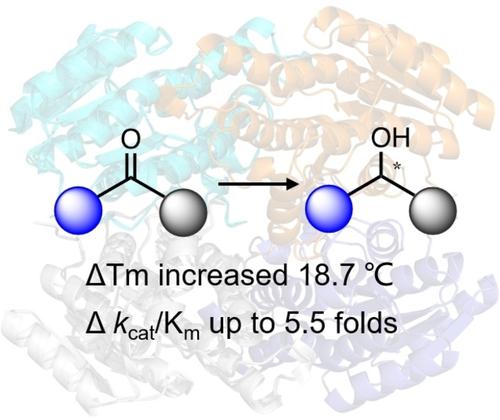
Engineering the subunit interface of Leifsonia alcohol dehydrogenase (LnADH) yielded thermostability, and catalytic efficiency significantly improved in the mutant T100R/S148I. T100R/S148I can efficiently convert various ketone substrates into nearly optically pure chiral alcohols at elevated temperature in a very low enzyme-to-substrate ratio (1 : 500).

中文翻译:

通过增强亚基相互作用来工程化 Leifsonia 酒精脱氢酶以提高热稳定性和催化效率
对Leifsonia醇脱氢酶 (LnADH)的亚基界面进行工程改造后,突变体 T100R/S148I 的热稳定性和催化效率显着提高。T100R/S148I 可以在高温下以非常低的酶与底物比 (1:500) 有效地将各种酮底物转化为几乎光学纯的手性醇。

更新日期:2021-11-16

中文翻译:

通过增强亚基相互作用来工程化 Leifsonia 酒精脱氢酶以提高热稳定性和催化效率
对Leifsonia醇脱氢酶 (LnADH)的亚基界面进行工程改造后,突变体 T100R/S148I 的热稳定性和催化效率显着提高。T100R/S148I 可以在高温下以非常低的酶与底物比 (1:500) 有效地将各种酮底物转化为几乎光学纯的手性醇。



























 京公网安备 11010802027423号
京公网安备 11010802027423号