当前位置:
X-MOL 学术
›
J. Raman Spectrosc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Deep-UV resonance Raman spectroscopy of hydrated and dehydrated model α-helical transmembrane peptides in liposomes
Journal of Raman Spectroscopy ( IF 2.5 ) Pub Date : 2021-09-21 , DOI: 10.1002/jrs.6252 Xing Wei 1 , Renee D. Jiji 1 , Anahita Zare 1 , Bryan Lada 1 , Xiyang Li 1 , C. Michael Greenlief 1
Journal of Raman Spectroscopy ( IF 2.5 ) Pub Date : 2021-09-21 , DOI: 10.1002/jrs.6252 Xing Wei 1 , Renee D. Jiji 1 , Anahita Zare 1 , Bryan Lada 1 , Xiyang Li 1 , C. Michael Greenlief 1
Affiliation
|
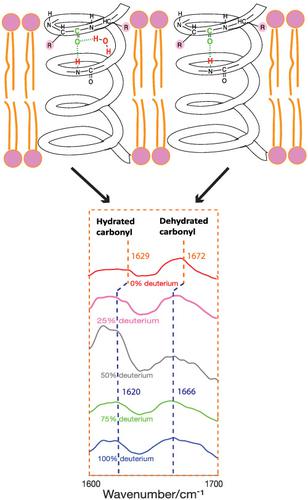
Water hydrogen bonding (H-bonding) to α-helical transmembrane (TM) peptides is fundamental to better understand the behavior and function of α-helical peptides, disease pathways, and the development of new drugs. Deep-UV resonance Raman (dUVRR) spectroscopy is a non-destructive technique amenable to both lipophilic and aqueous environments, which is an excellent and convenient approach for studying water H-bonding (or water accessibility) to α-helical TM peptides in a membrane mimicking environment. The dUVRR results indicate that water molecules can access the lipid membrane and form H-bonds with carbonyl groups along α-helical backbones. Raman bands at ~1,629 and ~1,672 cm−1 can be used to monitor the hydration and dehydration conditions along TM α-helices. Two bands at ~1,300 and ~1,340 cm−1 are also potential characteristic features of the dehydration and hydration along the α-helices in a membrane environment.
中文翻译:

脂质体中水合和脱水模型α-螺旋跨膜肽的深紫外共振拉曼光谱
水与 α-螺旋跨膜 (TM) 肽的氢键 (H-bonding) 是更好地了解 α- 螺旋肽的行为和功能、疾病途径和新药开发的基础。深紫外共振拉曼 (dUVRR) 光谱是一种非破坏性技术,适用于亲脂性和水性环境,是研究膜中水 H 键(或水可及性)与 α-螺旋 TM 肽的一种出色且方便的方法模仿环境。dUVRR 结果表明水分子可以进入脂质膜并与沿α-螺旋骨架的羰基形成氢键。~1,629 和 ~1,672 cm -1的拉曼谱带可用于监测沿 TM α-螺旋的水合和脱水条件。~1,300 和 ~1,340 cm -1的两个波段 也是膜环境中沿α-螺旋脱水和水合的潜在特征。
更新日期:2021-09-21
中文翻译:

脂质体中水合和脱水模型α-螺旋跨膜肽的深紫外共振拉曼光谱
水与 α-螺旋跨膜 (TM) 肽的氢键 (H-bonding) 是更好地了解 α- 螺旋肽的行为和功能、疾病途径和新药开发的基础。深紫外共振拉曼 (dUVRR) 光谱是一种非破坏性技术,适用于亲脂性和水性环境,是研究膜中水 H 键(或水可及性)与 α-螺旋 TM 肽的一种出色且方便的方法模仿环境。dUVRR 结果表明水分子可以进入脂质膜并与沿α-螺旋骨架的羰基形成氢键。~1,629 和 ~1,672 cm -1的拉曼谱带可用于监测沿 TM α-螺旋的水合和脱水条件。~1,300 和 ~1,340 cm -1的两个波段 也是膜环境中沿α-螺旋脱水和水合的潜在特征。



























 京公网安备 11010802027423号
京公网安备 11010802027423号