Kidney International ( IF 19.6 ) Pub Date : 2021-09-22 , DOI: 10.1016/j.kint.2021.08.027 Brad H Rovin 1 , Richard Furie 2 , Y K Onno Teng 3 , Gabriel Contreras 4 , Ana Malvar 5 , Xueqing Yu 6 , Beulah Ji 7 , Yulia Green 7 , Tania Gonzalez-Rivera 8 , Damon Bass 8 , Jennifer Gilbride 9 , Chun-Hang Tang 9 , David A Roth 8
|
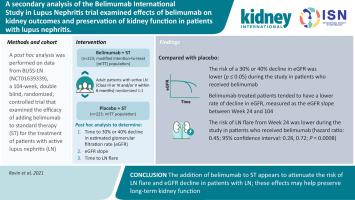
We performed a post hoc analysis of the Belimumab International Study in Lupus Nephritis (BLISS-LN), a Phase 3, multinational, double-blind, 104-week trial, in which 448 patients with lupus nephritis were randomized to receive intravenous belimumab 10 mg/kg or placebo with standard therapy (cyclophosphamide/azathioprine or mycophenolate mofetil). Add-on belimumab was found to be most effective in improving the primary efficacy kidney response and complete kidney response in patients with proliferative lupus nephritis and a baseline urine protein/creatinine ratio under 3 g/g. However, there was no observed improvement in the kidney response with belimumab treatment in patients with lupus nephritis and sub-epithelial deposits or with a baseline protein/creatinine ratio of 3 g/g or more. Belimumab significantly reduced the risk of kidney-related events or death and lupus nephritis flare in the overall population. Belimumab reduced the risk of a sustained 30% or 40% decline in estimated glomerular filtration rate (eGFR) versus standard treatment alone and attenuated the annual rate of eGFR decline in patients who remained on-study. Thus, our data suggest that the addition of belimumab to standard therapy could attenuate the risk of lupus nephritis flare and eGFR decline in a broad spectrum of patients with lupus nephritis.
中文翻译:

贝利木单抗国际狼疮性肾炎研究试验的二次分析检查了贝利木单抗对狼疮性肾炎患者肾脏结局和肾功能保持的影响
我们进行了事后贝利木单抗国际狼疮性肾炎研究 (BLISS-LN) 的分析,这是一项为期 3 期、多国、双盲、104 周的试验,其中 448 名狼疮性肾炎患者随机接受静脉注射贝利木单抗 10 mg/kg 或安慰剂标准治疗(环磷酰胺/硫唑嘌呤或霉酚酸酯)。发现在增殖性狼疮肾炎患者中,添加贝利单抗在改善主要疗效肾脏反应和完全肾脏反应方面最有效,并且基线尿蛋白/肌酐比低于 3 g/g。然而,在狼疮性肾炎和上皮下沉积或基线蛋白/肌酐比为 3 g/g 或更高的患者中,没有观察到用 belimumab 治疗的肾脏反应改善。Belimumab 显着降低了总体人群中肾脏相关事件或死亡和狼疮性肾炎发作的风险。与单独使用标准治疗相比,Belimumab 降低了估计肾小球滤过率 (eGFR) 持续下降 30% 或 40% 的风险,并降低了仍在研究患者中 eGFR 的年下降率。因此,我们的数据表明,在标准治疗中加入贝利木单抗可以降低广泛的狼疮性肾炎患者发生狼疮性肾炎发作和 eGFR 下降的风险。


























 京公网安备 11010802027423号
京公网安备 11010802027423号