当前位置:
X-MOL 学术
›
J. Mater. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
“Don’t eat me/eat me”-combined apoptotic body analogues for efficient targeted therapy of triple-negative breast cancer
Journal of Materials Chemistry B ( IF 7 ) Pub Date : 2021-08-31 , DOI: 10.1039/d1tb01116b Kailong Zhang 1 , Huanru Fu 1 , Chao Xing 2 , Yi Luo 1 , Fangfang Cheng 1 , Qiang Fu 1 , Yujie Huang 1 , Longxin Qiu 1
Journal of Materials Chemistry B ( IF 7 ) Pub Date : 2021-08-31 , DOI: 10.1039/d1tb01116b Kailong Zhang 1 , Huanru Fu 1 , Chao Xing 2 , Yi Luo 1 , Fangfang Cheng 1 , Qiang Fu 1 , Yujie Huang 1 , Longxin Qiu 1
Affiliation
|
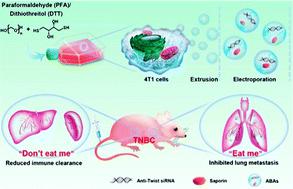
For the purpose of efficient targeted therapies, suppressing phagocytosis by a mononuclear phagocyte system (MPS), enhancing the “active” targeted delivery, and meeting clinical production criteria are extremely critical for engineering strategies of novel drug delivery systems. Herein, we used a chemically-induced membrane blebbing and extrusion combined method to induce triple-negative breast cancer (TNBC) cell apoptosis to secrete apoptotic body analogue (ABA) vesicles on a large scale for therapeutic drug delivery. After optimization, the ABAs have a desirable size, good biocompatibility, and long-term colloidal stability. Furthermore, ABAs present anti-phagocytosis (“don’t eat me”) and specific homologous targeting (“eat me”) capacities because of their inheritance of membrane proteins such as CD47 and cellular adhesion molecules from parent cells. After loading with toxic protein saporin and anti-twist siRNA, ABAs can significantly inhibit the growth and lung metastasis of TNBC in an orthotopic metastasis model due to their reduced clearance of immune organs, long circulation time, and enhanced targeted accumulation at the tumor sites. These results suggest the great potential of ABAs for targeted drug delivery therapy, in particular efficient TNBC treatment.
中文翻译:

“不要吃我/吃我”——联合凋亡小体类似物高效靶向治疗三阴性乳腺癌
为了有效的靶向治疗,抑制单核吞噬细胞系统(MPS)的吞噬作用,增强“主动”靶向递送,满足临床生产标准对于新型药物递送系统的工程策略至关重要。在此,我们使用化学诱导的膜起泡和挤压相结合的方法诱导三阴性乳腺癌(TNBC)细胞凋亡,从而大规模分泌凋亡小体类似物(ABA)囊泡用于治疗药物递送。优化后的ABA具有理想的尺寸、良好的生物相容性和长期的胶体稳定性。此外,ABA 具有抗吞噬作用(“不要吃我”)和特异性同源靶向(“吃我”)能力,因为它们继承了诸如 CD47 等膜蛋白和来自亲代细胞的细胞粘附分子。在负载有毒蛋白皂草素和抗扭曲 siRNA 后,ABA 可显着抑制 TNBC 在原位转移模型中的生长和肺转移,因为它们降低了免疫器官的清除率,延长了循环时间,并增强了肿瘤部位的靶向积累。这些结果表明 ABA 在靶向药物递送治疗中的巨大潜力,特别是有效的 TNBC 治疗。循环时间长,肿瘤部位靶向蓄积增强。这些结果表明 ABA 在靶向药物递送治疗中的巨大潜力,特别是有效的 TNBC 治疗。循环时间长,肿瘤部位靶向蓄积增强。这些结果表明 ABA 在靶向药物递送治疗中的巨大潜力,特别是有效的 TNBC 治疗。
更新日期:2021-09-22
中文翻译:

“不要吃我/吃我”——联合凋亡小体类似物高效靶向治疗三阴性乳腺癌
为了有效的靶向治疗,抑制单核吞噬细胞系统(MPS)的吞噬作用,增强“主动”靶向递送,满足临床生产标准对于新型药物递送系统的工程策略至关重要。在此,我们使用化学诱导的膜起泡和挤压相结合的方法诱导三阴性乳腺癌(TNBC)细胞凋亡,从而大规模分泌凋亡小体类似物(ABA)囊泡用于治疗药物递送。优化后的ABA具有理想的尺寸、良好的生物相容性和长期的胶体稳定性。此外,ABA 具有抗吞噬作用(“不要吃我”)和特异性同源靶向(“吃我”)能力,因为它们继承了诸如 CD47 等膜蛋白和来自亲代细胞的细胞粘附分子。在负载有毒蛋白皂草素和抗扭曲 siRNA 后,ABA 可显着抑制 TNBC 在原位转移模型中的生长和肺转移,因为它们降低了免疫器官的清除率,延长了循环时间,并增强了肿瘤部位的靶向积累。这些结果表明 ABA 在靶向药物递送治疗中的巨大潜力,特别是有效的 TNBC 治疗。循环时间长,肿瘤部位靶向蓄积增强。这些结果表明 ABA 在靶向药物递送治疗中的巨大潜力,特别是有效的 TNBC 治疗。循环时间长,肿瘤部位靶向蓄积增强。这些结果表明 ABA 在靶向药物递送治疗中的巨大潜力,特别是有效的 TNBC 治疗。



























 京公网安备 11010802027423号
京公网安备 11010802027423号