当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
B–N bond formation through palladium-catalyzed, microwave-assisted cross-coupling of nitrogen compounds with iodo-dodecaborate
Chemical Communications ( IF 4.9 ) Pub Date : 2021-09-13 , DOI: 10.1039/d1cc03215a Mahmoud K Al-Joumhawy 1 , Tarek Marei 1 , Akim Shmalko 1, 2 , Paula Cendoya 1 , Jair La Borde 1 , Detlef Gabel 1
Chemical Communications ( IF 4.9 ) Pub Date : 2021-09-13 , DOI: 10.1039/d1cc03215a Mahmoud K Al-Joumhawy 1 , Tarek Marei 1 , Akim Shmalko 1, 2 , Paula Cendoya 1 , Jair La Borde 1 , Detlef Gabel 1
Affiliation
|
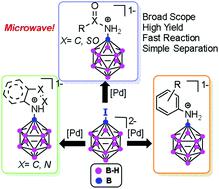
Substituted undecahydrido-closo-dodecaborates [B12H11NR2]2− have potential use in materials and drugs, but have presented a synthetic challenge. Microwave-assisted palladium-catalyzed amination of iodo-dodecaborate [B12H11I]2− allows mild and reproducible formation of B–N bonds with aromatic amines, HN-containing heteroaromatics, and amides. The reaction allows general access to amides, reproducible reactions to dodecaborate-substituted anilines, and, for the first time, the substitution of dodecaborate with HN-containing heterocycles.
中文翻译:

通过钯催化、微波辅助的氮化合物与碘-十二硼酸盐交叉偶联形成 B-N 键
取代undecahydrido-闭合碳-dodecaborates [B 12 ħ 11 NR 2 ] 2-具有在材料和药物的潜在用途,但已经提出了一种合成的挑战。微波辅助钯催化的碘十二硼酸盐 [B 12 H 11 I] 2-胺化允许与芳香胺、含 HN 的杂芳烃和酰胺温和且可重复地形成 B-N 键。该反应允许一般访问酰胺,可重现反应以十二硼酸盐取代的苯胺,并首次用含 HN 的杂环取代十二硼酸盐。
更新日期:2021-09-22
中文翻译:

通过钯催化、微波辅助的氮化合物与碘-十二硼酸盐交叉偶联形成 B-N 键
取代undecahydrido-闭合碳-dodecaborates [B 12 ħ 11 NR 2 ] 2-具有在材料和药物的潜在用途,但已经提出了一种合成的挑战。微波辅助钯催化的碘十二硼酸盐 [B 12 H 11 I] 2-胺化允许与芳香胺、含 HN 的杂芳烃和酰胺温和且可重复地形成 B-N 键。该反应允许一般访问酰胺,可重现反应以十二硼酸盐取代的苯胺,并首次用含 HN 的杂环取代十二硼酸盐。


























 京公网安备 11010802027423号
京公网安备 11010802027423号