当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Acid-catalyzed cleavage of C–C bonds enables atropaldehyde acetals as masked C2 electrophiles for organic synthesis
Chemical Communications ( IF 4.9 ) Pub Date : 2021-09-13 , DOI: 10.1039/d1cc04000f Shaomin Chen 1 , Minghao Li 1 , Yanlong Gu 1, 2, 3
Chemical Communications ( IF 4.9 ) Pub Date : 2021-09-13 , DOI: 10.1039/d1cc04000f Shaomin Chen 1 , Minghao Li 1 , Yanlong Gu 1, 2, 3
Affiliation
|
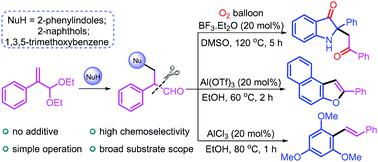
Acid-catalyzed tandem reactions of atropaldehyde acetals were established for the synthesis of three important molecules, 2,2-disubstituted indolin-3-ones, naphthofurans and stilbenes. The synthesis was realized using novel reaction cascades, which involved the same two initial steps: (i) SN2′ substitution, in which the atropaldehyde acted as an electrophile; and (ii) oxidative cleavage of the carbon–carbon bond of the generated phenylacetaldehyde-type products. Compared with literature methods, the present protocol not only avoided the use of expensive noble metal catalysts, but also enabled a simple operation.
中文翻译:

C-C键的酸催化裂解使阿托醛缩醛成为有机合成的掩蔽C2亲电试剂
阿托醛缩醛的酸催化串联反应被建立用于合成三个重要分子,2,2-二取代 indolin-3-ones、萘并呋喃和芪。该合成是使用新的反应级联反应实现的,其中涉及相同的两个初始步骤:(i) S N 2' 取代,其中阿托醛充当亲电子试剂;(ii) 生成的苯乙醛类产物的碳-碳键的氧化裂解。与文献方法相比,本协议不仅避免使用昂贵的贵金属催化剂,而且操作简单。
更新日期:2021-09-22
中文翻译:

C-C键的酸催化裂解使阿托醛缩醛成为有机合成的掩蔽C2亲电试剂
阿托醛缩醛的酸催化串联反应被建立用于合成三个重要分子,2,2-二取代 indolin-3-ones、萘并呋喃和芪。该合成是使用新的反应级联反应实现的,其中涉及相同的两个初始步骤:(i) S N 2' 取代,其中阿托醛充当亲电子试剂;(ii) 生成的苯乙醛类产物的碳-碳键的氧化裂解。与文献方法相比,本协议不仅避免使用昂贵的贵金属催化剂,而且操作简单。


























 京公网安备 11010802027423号
京公网安备 11010802027423号