当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Influence of the particle size on selective 2-propanol gas-phase oxidation over Co3O4 nanospheres
Catalysis Science & Technology ( IF 5 ) Pub Date : 2021-09-13 , DOI: 10.1039/d1cy00944c Tobias Falk 1 , Sven Anke 1 , Hamidreza Hajiyani 2 , Sascha Saddeler 3 , Stephan Schulz 3 , Rossitza Pentcheva 2 , Baoxiang Peng 1, 4 , Martin Muhler 1, 4
Catalysis Science & Technology ( IF 5 ) Pub Date : 2021-09-13 , DOI: 10.1039/d1cy00944c Tobias Falk 1 , Sven Anke 1 , Hamidreza Hajiyani 2 , Sascha Saddeler 3 , Stephan Schulz 3 , Rossitza Pentcheva 2 , Baoxiang Peng 1, 4 , Martin Muhler 1, 4
Affiliation
|
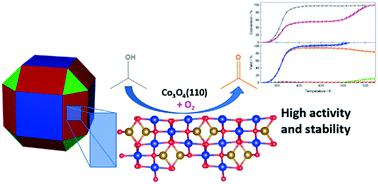
Co3O4 nanospheres with a mean diameter of 19 nm were applied in the selective oxidation of 2-propanol to acetone in the gas phase. Compared with 9 nm spheres, the 19 nm spheres exhibited superior catalytic activity and stability with 100% selectivity to acetone up to 500 K. Transmission electron microscopy, N2 physisorption, 2-propanol and O2 temperature-programmed desorption, and 2-propanol temperature-programmed surface reaction in O2 were applied to characterize the bulk and surface properties. Despite the smaller specific surface area (35 m2 g−1), an increased 2-propanol adsorption capacity was observed for the larger nanospheres ascribed to a preferential (110) surface orientation. Temperature-programmed oxidation experiments after reaction showed multilayer coke deposition and severe reduction of the Co3O4 surface, but excellent stability was maintained at 430 K using the 19 nm spheres in a steady-state oxidation experiment for 100 h with only 10% loss of the initial activity. The good agreement of the 2-propanol decomposition profiles indicates that the superior activity is caused by the enhanced interaction of the larger nanospheres with O2. A Mars–van Krevelen mechanism on the (110) surface was identified by density functional theory calculations with a Hubbard U term, favoring faster reoxidation compared with the (100) surface predominantly exposed by the 9 nm spheres.
中文翻译:

粒径对 Co3O4 纳米球选择性 2-丙醇气相氧化的影响
平均直径为19 nm的Co 3 O 4纳米球用于在气相中将2-丙醇选择性氧化为丙酮。与 9 nm 球相比,19 nm 球表现出优异的催化活性和稳定性,对丙酮的选择性高达 500 K。 透射电子显微镜、N 2物理吸附、2-丙醇和 O 2 程序升温脱附以及 2-丙醇O 2中的程序升温表面反应被用于表征体积和表面性质。尽管比表面积较小 (35 m 2 g -1),由于优先的 (110) 表面取向,观察到较大的纳米球的 2-丙醇吸附容量增加。反应后的程序升温氧化实验显示多层焦炭沉积和 Co 3 O 4表面的严重还原,但在 430 K 下使用 19 nm 球体在稳态氧化实验中保持了 100 小时的优异稳定性,仅损失 10%最初的活动。2-丙醇分解曲线的良好一致性表明优异的活性是由较大的纳米球与O 2的增强相互作用引起的。使用 Hubbard U 的密度泛函理论计算确定了 (110) 表面上的 Mars-van Krevelen 机制 术语,与主要由 9 nm 球体暴露的 (100) 表面相比,有利于更快的再氧化。
更新日期:2021-09-22
中文翻译:

粒径对 Co3O4 纳米球选择性 2-丙醇气相氧化的影响
平均直径为19 nm的Co 3 O 4纳米球用于在气相中将2-丙醇选择性氧化为丙酮。与 9 nm 球相比,19 nm 球表现出优异的催化活性和稳定性,对丙酮的选择性高达 500 K。 透射电子显微镜、N 2物理吸附、2-丙醇和 O 2 程序升温脱附以及 2-丙醇O 2中的程序升温表面反应被用于表征体积和表面性质。尽管比表面积较小 (35 m 2 g -1),由于优先的 (110) 表面取向,观察到较大的纳米球的 2-丙醇吸附容量增加。反应后的程序升温氧化实验显示多层焦炭沉积和 Co 3 O 4表面的严重还原,但在 430 K 下使用 19 nm 球体在稳态氧化实验中保持了 100 小时的优异稳定性,仅损失 10%最初的活动。2-丙醇分解曲线的良好一致性表明优异的活性是由较大的纳米球与O 2的增强相互作用引起的。使用 Hubbard U 的密度泛函理论计算确定了 (110) 表面上的 Mars-van Krevelen 机制 术语,与主要由 9 nm 球体暴露的 (100) 表面相比,有利于更快的再氧化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号