当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Nickel oxide regulating surface oxygen to promote formaldehyde oxidation on manganese oxide catalysts
Catalysis Science & Technology ( IF 5 ) Pub Date : 2021-09-02 , DOI: 10.1039/d1cy01490k Hailin Zhao 1 , Jie Tang 2 , Zengyuan Li 1 , Jie Yang 1 , Hao Liu 1 , Li Wang 1 , Yao Cui 2 , Wangcheng Zhan 1 , Yanglong Guo 1 , Yun Guo 1
Catalysis Science & Technology ( IF 5 ) Pub Date : 2021-09-02 , DOI: 10.1039/d1cy01490k Hailin Zhao 1 , Jie Tang 2 , Zengyuan Li 1 , Jie Yang 1 , Hao Liu 1 , Li Wang 1 , Yao Cui 2 , Wangcheng Zhan 1 , Yanglong Guo 1 , Yun Guo 1
Affiliation
|
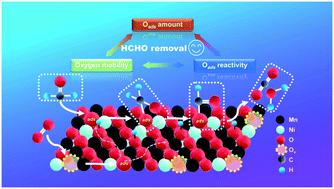
Catalytic oxidation is the most effective method to eliminate in-door formaldehyde, and Mn-based catalysts with low cost and high activity have drawn great attention for this reaction. Herein, p-type semiconductor NiO doped MnOx catalysts were prepared by an environmentally friendly oxalate co-precipitation method. The doped Ni species entered the lattice of MnOx to form amorphous Ni–Mn composite oxide/NiO and increased the ratio of surface Mn4+ and total amount of surface active oxygen simultaneously with the increase of NiO content in the form of a volcano curve, which directly correlated to the abilities for adsorption and oxidation of formaldehyde. Among them, 0.2NiO–MnOx (Ni/(Ni + Mn) = 0.2) showed the highest activity, 300 ppm of formaldehyde can be completely eliminated at 98 °C in a 2.5 vol% H2O-containing atmosphere, and the corresponding specific reaction rate was about 2.9 times higher than that of pure MnOx. Meanwhile, the enhanced migration of oxygen species over NiO–MnOx catalysts also promoted the replenishment of surface active oxygen that was consumed in the reaction process, which kept the activity of 0.2NiO–MnOx stable during the continuous reaction cycles and 26 h long-term stability test. Our study showed that utilizing NiO to regulate the reactivity and amount of surface oxygen species was an efficient way to improve the intrinsic catalytic performance of MnOx.
中文翻译:

氧化镍调节表面氧以促进氧化锰催化剂上的甲醛氧化
催化氧化是消除室内甲醛最有效的方法,低成本、高活性的锰基催化剂因该反应而备受关注。在此,采用环保草酸盐共沉淀法制备了p 型半导体 NiO 掺杂的 MnO x催化剂。掺杂的 Ni 物种进入 MnO x的晶格形成非晶 Ni-Mn 复合氧化物/NiO,并随着NiO 含量的增加以火山曲线的形式增加了表面 Mn 4+和表面活性氧总量的比率,这与甲醛的吸附和氧化能力直接相关。其中,0.2NiO,MnO的X(Ni/(Ni + Mn) = 0.2) 表现出最高的活性,在 98°C 下在 2.5 vol% H 2 O 的气氛中可以完全消除 300 ppm 的甲醛,相应的比反应速率约为 2.9 倍高于纯 MnO x。同时,氧物种在 NiO-MnO x催化剂上迁移的增强也促进了反应过程中消耗的表面活性氧的补充,这使得 0.2NiO-MnO x的活性在连续反应循环和 26 小时内保持稳定。 - 长期稳定性测试。我们的研究表明,利用 NiO 调节表面氧物种的反应性和数量是提高 MnO x内在催化性能的有效方法.
更新日期:2021-09-22
中文翻译:

氧化镍调节表面氧以促进氧化锰催化剂上的甲醛氧化
催化氧化是消除室内甲醛最有效的方法,低成本、高活性的锰基催化剂因该反应而备受关注。在此,采用环保草酸盐共沉淀法制备了p 型半导体 NiO 掺杂的 MnO x催化剂。掺杂的 Ni 物种进入 MnO x的晶格形成非晶 Ni-Mn 复合氧化物/NiO,并随着NiO 含量的增加以火山曲线的形式增加了表面 Mn 4+和表面活性氧总量的比率,这与甲醛的吸附和氧化能力直接相关。其中,0.2NiO,MnO的X(Ni/(Ni + Mn) = 0.2) 表现出最高的活性,在 98°C 下在 2.5 vol% H 2 O 的气氛中可以完全消除 300 ppm 的甲醛,相应的比反应速率约为 2.9 倍高于纯 MnO x。同时,氧物种在 NiO-MnO x催化剂上迁移的增强也促进了反应过程中消耗的表面活性氧的补充,这使得 0.2NiO-MnO x的活性在连续反应循环和 26 小时内保持稳定。 - 长期稳定性测试。我们的研究表明,利用 NiO 调节表面氧物种的反应性和数量是提高 MnO x内在催化性能的有效方法.


























 京公网安备 11010802027423号
京公网安备 11010802027423号