当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Solvate electrolytes for Li and Na batteries: structures, transport properties, and electrochemistry
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2021-09-15 , DOI: 10.1039/d1cp02946k Yosuke Ugata 1 , Keisuke Shigenobu 1 , Ryoichi Tatara 2, 3 , Kazuhide Ueno 1, 4 , Masayoshi Watanabe 4 , Kaoru Dokko 1, 3, 4
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2021-09-15 , DOI: 10.1039/d1cp02946k Yosuke Ugata 1 , Keisuke Shigenobu 1 , Ryoichi Tatara 2, 3 , Kazuhide Ueno 1, 4 , Masayoshi Watanabe 4 , Kaoru Dokko 1, 3, 4
Affiliation
|
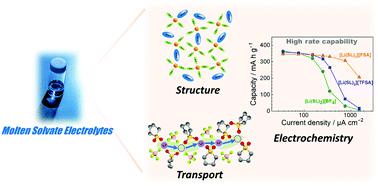
Polar solvents dissolve Li and Na salts at high concentrations and are used as electrolyte solutions for batteries. The solvents interact strongly with the alkali metal cations to form complexes in the solution. The activity (concentration) of the uncoordinated solvent decreases as the salt concentration is increased. At extremely high salt concentrations, all the solvent molecules are involved in the coordination of the ions and form the solvates of the salts. In this article, we review the structures, transport properties, and electrochemistry of Li/Na salt solvates. In molten solvates, the activity of the uncoordinated solvent is negligible; this is the main origin of their peculiar characteristics, such as high thermal stability, wide electrochemical window, and unique ion transport. In addition, the solvent activity greatly influences the electrochemical reactions in Li/Na batteries. We highlight the attractive features of molten solvates as promising electrolytes for next-generation batteries.
中文翻译:

锂和钠电池的溶剂化电解质:结构、传输特性和电化学
极性溶剂可溶解高浓度的锂盐和钠盐,并用作电池的电解质溶液。溶剂与碱金属阳离子强烈相互作用,在溶液中形成络合物。未配位溶剂的活性(浓度)随着盐浓度的增加而降低。在极高的盐浓度下,所有溶剂分子都参与离子的配位并形成盐的溶剂化物。在本文中,我们回顾了 Li/Na 盐溶剂化物的结构、传输特性和电化学。在熔融溶剂化物中,未配位溶剂的活性可以忽略不计;这是它们特殊特性的主要来源,例如高热稳定性、宽电化学窗口和独特的离子传输。此外,溶剂活性极大地影响锂/钠电池中的电化学反应。我们强调了熔融溶剂化物作为下一代电池有前途的电解质的吸引力。
更新日期:2021-09-22
中文翻译:

锂和钠电池的溶剂化电解质:结构、传输特性和电化学
极性溶剂可溶解高浓度的锂盐和钠盐,并用作电池的电解质溶液。溶剂与碱金属阳离子强烈相互作用,在溶液中形成络合物。未配位溶剂的活性(浓度)随着盐浓度的增加而降低。在极高的盐浓度下,所有溶剂分子都参与离子的配位并形成盐的溶剂化物。在本文中,我们回顾了 Li/Na 盐溶剂化物的结构、传输特性和电化学。在熔融溶剂化物中,未配位溶剂的活性可以忽略不计;这是它们特殊特性的主要来源,例如高热稳定性、宽电化学窗口和独特的离子传输。此外,溶剂活性极大地影响锂/钠电池中的电化学反应。我们强调了熔融溶剂化物作为下一代电池有前途的电解质的吸引力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号