当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Using a Chromatographic Pseudophase Model To Elucidate the Mechanism of Olefin Separation by Silver(I) Ions in Ionic Liquids
Analytical Chemistry ( IF 7.4 ) Pub Date : 2021-09-22 , DOI: 10.1021/acs.analchem.1c02885 Philip Eor 1, 2 , Jared L Anderson 1, 2
Analytical Chemistry ( IF 7.4 ) Pub Date : 2021-09-22 , DOI: 10.1021/acs.analchem.1c02885 Philip Eor 1, 2 , Jared L Anderson 1, 2
Affiliation
|
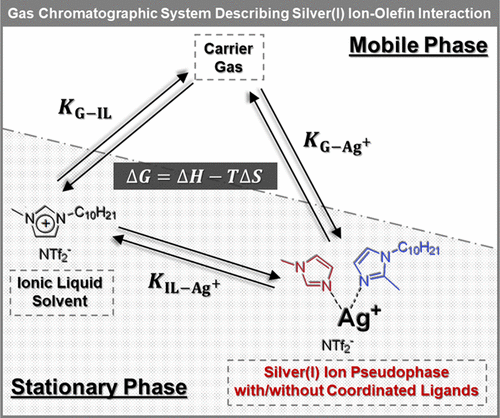
Silver(I) ions undergo selective olefin complexation and have been utilized in various olefin/paraffin separation techniques such as argentation chromatography and facilitated transport membranes. Ionic liquids (ILs) are solvents known for their low vapor pressure, high thermal stability, low melting points, and ability to promote a favorable solvation environment for silver(I) ion–olefin interactions. To develop highly selective separation systems, a fundamental understanding of analyte partitioning to the stationary phase and the thermodynamic driving forces behind solvation is required. In this study, a chromatographic model treating silver(I) ions as a pseudophase is constructed and employed for the first time to investigate the olefin separation mechanism in silver(I) salt/IL mixtures. Stationary phases containing varying amounts of noncoordinated silver(I) salt ([Ag+][NTf2–]) dissolved in the 1-decyl-3-methylimidazolium bis[(trifluoromethyl)sulfonyl]imide ([C10MIM+][NTf2–]) IL are utilized to determine the partition coefficients of various analytes including alkanes, alkenes, alkynes, aromatics, aldehyde, esters, and ketones. As ligand coordination to silver(I) ions is known to lower its olefin complexation capability, this study also examines two different types of coordinated silver(I) ion pseudophases, namely, monocoordinated silver(I) salt ([Ag+(1-decyl-2-methylimidazole, DMIM)][NTf2–]) and dicoordinated silver(I) salt ([Ag+(1-methylimidazole, MIM)(DMIM)][NTf2–]). The extent of olefin partitioning to the coordinated silver(I) ion pseudophases over the carrier gas and IL decreased by up to two orders of magnitude. Values for enthalpy, entropy, and free energy of solvation were determined for the three silver(I) ion-containing systems. Olefin retention was observed to be enthalpically dominated, while ligand coordination to the silver(I) ion pseudophase resulted in variations for both enthalpic and entropic contributions to the free energy of solvation. The developed model can be used to study chemical changes that occur in silver(I) ions over time as well as identify optimal silver(I) salt/IL mixtures that yield high olefin selectivity.
中文翻译:

使用色谱假相模型阐明离子液体中银 (I) 离子分离烯烃的机制
银 (I) 离子经历选择性烯烃络合,并已用于各种烯烃/石蜡分离技术,例如银化色谱和促进传输膜。离子液体 (IL) 是一种溶剂,以其低蒸气压、高热稳定性、低熔点以及为银 (I) 离子-烯烃相互作用促进有利的溶剂化环境的能力而闻名。为了开发高选择性分离系统,需要对分析物分配到固定相和溶剂化背后的热力学驱动力有基本的了解。在本研究中,首次构建了以银 (I) 离子为假相的色谱模型,并首次用于研究银 (I) 盐 / IL 混合物中的烯烃分离机制。+ ][NTf 2 – ]) 溶解在 1-decyl-3-methylimidazolium 双[(trifluoromethyl)sulfonyl]imide ([C 10 MIM + ][NTf 2 – ]) IL 中,用于确定各种分析物的分配系数包括烷烃、烯烃、炔烃、芳烃、醛、酯和酮。由于已知配体与银 (I) 离子的配位会降低其烯烃络合能力,因此本研究还检查了两种不同类型的配位银 (I) 离子假相,即单配位银 (I) 盐 ([Ag + (1-decyl -2-甲基咪唑,DMIM)][NTf 2 – ]) 和双配位银 (I) 盐([Ag + (1-methylimidazole, MIM)(DMIM)][NTf2 – ])。烯烃分配到载气和 IL 上配位的银 (I) 离子假相的程度减少了两个数量级。测定了三种含银 (I) 离子系统的焓、熵和溶剂化自由能的值。观察到烯烃保留以焓为主,而银(I)离子假相的配位配位导致对溶剂化自由能的焓和熵贡献的变化。开发的模型可用于研究银 (I) 离子随时间发生的化学变化,以及确定可产生高烯烃选择性的最佳银 (I) 盐/IL 混合物。
更新日期:2021-10-06
中文翻译:

使用色谱假相模型阐明离子液体中银 (I) 离子分离烯烃的机制
银 (I) 离子经历选择性烯烃络合,并已用于各种烯烃/石蜡分离技术,例如银化色谱和促进传输膜。离子液体 (IL) 是一种溶剂,以其低蒸气压、高热稳定性、低熔点以及为银 (I) 离子-烯烃相互作用促进有利的溶剂化环境的能力而闻名。为了开发高选择性分离系统,需要对分析物分配到固定相和溶剂化背后的热力学驱动力有基本的了解。在本研究中,首次构建了以银 (I) 离子为假相的色谱模型,并首次用于研究银 (I) 盐 / IL 混合物中的烯烃分离机制。+ ][NTf 2 – ]) 溶解在 1-decyl-3-methylimidazolium 双[(trifluoromethyl)sulfonyl]imide ([C 10 MIM + ][NTf 2 – ]) IL 中,用于确定各种分析物的分配系数包括烷烃、烯烃、炔烃、芳烃、醛、酯和酮。由于已知配体与银 (I) 离子的配位会降低其烯烃络合能力,因此本研究还检查了两种不同类型的配位银 (I) 离子假相,即单配位银 (I) 盐 ([Ag + (1-decyl -2-甲基咪唑,DMIM)][NTf 2 – ]) 和双配位银 (I) 盐([Ag + (1-methylimidazole, MIM)(DMIM)][NTf2 – ])。烯烃分配到载气和 IL 上配位的银 (I) 离子假相的程度减少了两个数量级。测定了三种含银 (I) 离子系统的焓、熵和溶剂化自由能的值。观察到烯烃保留以焓为主,而银(I)离子假相的配位配位导致对溶剂化自由能的焓和熵贡献的变化。开发的模型可用于研究银 (I) 离子随时间发生的化学变化,以及确定可产生高烯烃选择性的最佳银 (I) 盐/IL 混合物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号