当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enantioselective Construction of 1H-Isoindoles Containing Tri- and Difluoromethylated Quaternary Stereogenic Centers via Palladium-Catalyzed C–H Bond Imidoylation
ACS Catalysis ( IF 12.9 ) Pub Date : 2021-09-22 , DOI: 10.1021/acscatal.1c03682 Jian Wang 1 , Lianjie Li 1 , Minxue Chai 1 , Shumin Ding 1 , Jing Li 2, 3, 4 , Yongjia Shang 1 , Haixia Zhao 1 , Dan Li 1 , Qiang Zhu 2, 3, 4
ACS Catalysis ( IF 12.9 ) Pub Date : 2021-09-22 , DOI: 10.1021/acscatal.1c03682 Jian Wang 1 , Lianjie Li 1 , Minxue Chai 1 , Shumin Ding 1 , Jing Li 2, 3, 4 , Yongjia Shang 1 , Haixia Zhao 1 , Dan Li 1 , Qiang Zhu 2, 3, 4
Affiliation
|
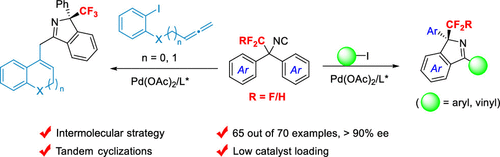
An intermolecular enantioselective synthesis of 1H-isoindoles containing tri- and difluoromethylated quaternary stereogenic centers through a palladium-catalyzed desymmetric C–H bond imidoylation has been developed. α,α-Diaryl tri- and difluoroethylated isocyanides acted as powerful precursors of chiral 1H-isoindoles, in which the fluoroalkyl group was proven to play a crucial role in both the stereochemistry and reaction efficiency. In addition, an allene insertion cascade was realized, offering rapid access to diverse C1-tethered bis-heterocyclic scaffolds with good yields and high enantioselectivities. The reactions proceeded smoothly under mild conditions with low catalyst loadings.
中文翻译:

通过钯催化的 C-H 键亚胺酰化对含有三和二氟甲基化四元立体中心的 1H-异吲哚进行对映选择性构建
已经开发了一种通过钯催化的去对称 C-H 键亚胺酰化作用合成含有三和二氟甲基化季立体中心的 1 H-异吲哚的分子间对映选择性合成。α,α-二芳基三和二氟乙基化异氰化物是手性 1 H-异吲哚的强大前体,其中氟烷基被证明在立体化学和反应效率中都起着至关重要的作用。此外,实现了丙二烯插入级联,可快速获得具有良好产率和高对映选择性的各种 C1 系链双杂环支架。反应在温和条件下顺利进行,催化剂负载量低。
更新日期:2021-10-01
中文翻译:

通过钯催化的 C-H 键亚胺酰化对含有三和二氟甲基化四元立体中心的 1H-异吲哚进行对映选择性构建
已经开发了一种通过钯催化的去对称 C-H 键亚胺酰化作用合成含有三和二氟甲基化季立体中心的 1 H-异吲哚的分子间对映选择性合成。α,α-二芳基三和二氟乙基化异氰化物是手性 1 H-异吲哚的强大前体,其中氟烷基被证明在立体化学和反应效率中都起着至关重要的作用。此外,实现了丙二烯插入级联,可快速获得具有良好产率和高对映选择性的各种 C1 系链双杂环支架。反应在温和条件下顺利进行,催化剂负载量低。


























 京公网安备 11010802027423号
京公网安备 11010802027423号