Journal of Hazardous Materials ( IF 13.6 ) Pub Date : 2021-09-20 , DOI: 10.1016/j.jhazmat.2021.127272 Min Chen 1 , Qiwu Zhang 1 , Lin Jiang 1 , Huimin Hu 1 , Chao Wang 1 , Zhao Li 2
|
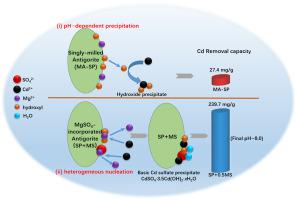
Utilization of natural clay minerals for the treatment of heavy metal cadmium contamination is appealing as the affordable and readily accessible raw materials. However, the low reactivity of natural serpentine limits its practical application for Cd removal. In the present study, mechanochemical activation of antigorite-type serpentine (SP) as example was introduced to enhance its removal capacity for heavy metal of cadmium high enough for practical use. It was found ball-milling at 600 rpm for 60 min for antigorite resulted in the increased release of hydroxyl group to facilitate the precipitation of Cd2+, giving a capacity of 27.4 mg/g for the treatment of 100 mg/L Cd2+ for 120 min at room temperature, which was 10 times higher than that of the pristine antigorite (2.5 mg/g). More significantly, magnesium sulfate (MgSO4, MS) was introduced for the first time to process antigorite, thus to form MgSO4-incorporated antigorite. As a result, the removal capacity for Cd2+ was dramatically increased to 239.7 mg/g with the equal antigorite dosage (the molar ratio of SP/MS = 1:0.5), which is also much higher than the other reported clay minerals. Results showed that, MgSO4 incorporation promoted the reactivity of antigorite and provided numerous SO42− active sites, which allowed the heterogeneous nucleation of basic cadmium sulfate (CdSO4·3.5 Cd(OH)2·xH2O) precipitate on antigorite, therefore not requiring high alkalinity support as the conventional formation of cadmium hydroxide does. Correspondingly, under the new mechanism, the Cd precipitation could take place in a wide pH range, even from pH 3.0, which was a rarely reported phenomenon happening on natural minerals. Based on these findings, this study demonstrated the effectiveness of mechanochemical incorporation of sulfate for enhancing the Cd2+ removal capacity of serpentine, as well as the high efficiency of new pathway for Cd2+ precipitation. Moreover, the potential of low-cost serpentine as alternative stabilizers for the highly-effective remediation of heavy metal contamination may be expected.
中文翻译:

将硫酸镁机械化学结合到叶蛇纹石中,为从弱酸性溶液中有效沉淀镉离子提供活性成核位点
利用天然粘土矿物处理重金属镉污染是一种价格合理且易于获得的原材料,具有吸引力。然而,天然蛇纹石的低反应性限制了其在去除镉方面的实际应用。在本研究中,以叶蛇纹石型蛇纹石(SP)的机械化学活化为例,以提高其对重金属镉的去除能力,使其达到实用的水平。发现在 600 rpm 下球磨 60 分钟的叶蛇纹石导致羟基释放增加,有利于 Cd 2+ 的沉淀,处理100 mg/L Cd 2+的容量为 27.4 mg/g在室温下放置 120 分钟,这是原始叶蛇纹石 (2.5 mg/g) 的 10 倍。更重要的是,首次引入硫酸镁(MgSO 4 ,MS)对叶蛇纹石进行加工,从而形成了掺有MgSO 4的叶蛇纹石。结果,在叶蛇纹石用量相同(SP/MS 的摩尔比 = 1:0.5)的情况下,Cd 2+的去除能力显着提高到 239.7 mg/g,这也远高于其他报道的粘土矿物。结果表明,MgSO 4的掺入促进了叶蛇纹石的反应性,并提供了大量的 SO 4 2-活性位点,这使得碱性硫酸镉(CdSO 4·3.5 Cd(OH) 2 · x H 2 O) 沉淀在叶蛇纹石上,因此不需要像氢氧化镉的常规形成那样需要高碱度载体。相应地,在新机制下,Cd 的沉淀可以在较宽的 pH 范围内发生,甚至从 pH 3.0 开始,这在天然矿物上是很少报道的现象。基于这些发现,本研究证明了硫酸盐的机械化学掺入对提高蛇纹石Cd 2+去除能力的有效性,以及Cd 2+新途径的高效性。沉淀。此外,可以预期低成本蛇纹石作为替代稳定剂用于高效修复重金属污染的潜力。


























 京公网安备 11010802027423号
京公网安备 11010802027423号