Journal of Environmental Chemical Engineering ( IF 7.7 ) Pub Date : 2021-09-20 , DOI: 10.1016/j.jece.2021.106394 Prangan Duarah 1 , Dibyajyoti Haldar 2 , VSK Yadav 3 , Mihir Kumar Purkait 3
|
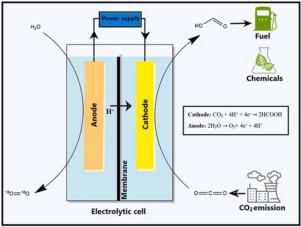
The utilization of CO2 to produce formic acid (HCOOH) is regarded as an acceptable and efficient approach for mitigating the negative effects of gaseous emissions from chemical industries and other anthropogenic activities. Electrochemical reduction (ECR) is a method for converting CO2 to HCOOH in which electrocatalysts, electrodes, and electrolytes all play a vital role. Despite considerable advancements in CO2ECR, its efficiency is still hampered by a number of difficulties, including high electrocatalyst costs, selectivity toward HCOOH production, poor electrode surface area, and reactor mass transfer limitation. Numerous researches have been performed in recent decades to alleviate such constraints. The current review focuses on the progress in the production of HCOOH through CO2ECR. The involvement of the electrocatalyst, electrode and electrolyte in the ECR process is thoroughly examined. Furthermore, the impact of different parameters such as pressure, temperature, and reactor design are thoroughly analyzed. Further, the commercial aspects and techno-economic analysis of the utilization of CO2 are featured to highlight the process feasibility. Finally, this paper is concluded by discussing the challenges that are frequently encountered during the ECR process for HCOOH production with an aspiration of futuristic developments. The readers of this paper will be highly benefited by acquiring comprehensive knowledge on the ECR mechanism for HCOOH production.
中文翻译:

CO2电化学还原为甲酸的进展:当前趋势和未来前景回顾
利用 CO 2生产甲酸 (HCOOH) 被认为是减轻化学工业和其他人为活动的气体排放的负面影响的可接受且有效的方法。电化学还原 (ECR) 是一种将 CO 2转化为 HCOOH 的方法,其中电催化剂、电极和电解质都起着至关重要的作用。尽管 CO 2取得了长足的进步ECR,其效率仍然受到许多困难的阻碍,包括高电催化剂成本、对 HCOOH 生产的选择性、较差的电极表面积和反应器传质限制。近几十年来,已经进行了大量研究以减轻这种限制。目前的审查重点是通过 CO 2 ECR生产 HCOOH 的进展。电催化剂、电极和电解质在 ECR 过程中的参与被彻底检查。此外,还彻底分析了压力、温度和反应器设计等不同参数的影响。此外,CO 2的利用的商业方面和技术经济分析以突出工艺可行性为特色。最后,本文通过讨论在 HCOOH 生产的 ECR 过程中经常遇到的挑战以及未来发展的愿望来结束。通过全面了解 HCOOH 生产的 ECR 机制,本文的读者将受益匪浅。


























 京公网安备 11010802027423号
京公网安备 11010802027423号