当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
High Catalytic Activities of RENi5–xAlx (RE = La, Er) and Low Activity of Mg2Ni Following Hydrogen Uptake: The Role of Absorbed Hydrogen
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2021-09-21 , DOI: 10.1021/acs.jpcc.1c06484 Ryota Tsukuda 1, 2 , Takayuki Kojima 1, 3 , Ya Xu 4 , Chikashi Nishimura 4 , Marian Krajčí 5 , Satoshi Kameoka 1
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2021-09-21 , DOI: 10.1021/acs.jpcc.1c06484 Ryota Tsukuda 1, 2 , Takayuki Kojima 1, 3 , Ya Xu 4 , Chikashi Nishimura 4 , Marian Krajčí 5 , Satoshi Kameoka 1
Affiliation
|
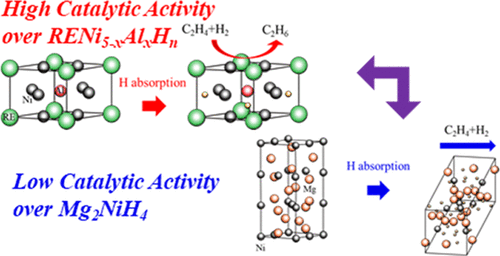
Catalysis by intermetallic compounds (IMCs) has recently received significant attention, and IMCs that are also capable of storing hydrogen, including RENi5–xAlx (RE = La, Er) and Mg2Ni, have been employed as catalysts for the hydrogenation of ethylene. RENi5–xAlx with absorbed hydrogen exhibits improved catalytic activity and high levels of ethylene conversion. The reaction rate for RENi5–xAlx with absorbed hydrogen is higher than those for pure Ni and Cu and close to that for Pd. Pulsed gas tests have demonstrated that the presence of absorbed hydrogen is essential for high catalytic activity. In this work, the role of absorbed hydrogen in catalytic reactions was assessed by performing the trial using deuterium. It was found that absorbed deuterium in ErNi3.75Al1.25Dn undergoes very little reaction with ethylene. A novel activation mechanism which is different from the reaction of absorbed hydrogen directly with C2H4 was proposed. This mechanism was investigated based on adsorption experiments and density functional theory calculations of electronic states. It was concluded that weakly adsorbed ethylene and hydrogen are more reactive on the hydride surface than on the matrix surface with no absorbed hydrogen, leading to an increase in catalytic activity. In contrast, the hydride of Mg2Ni, Mg2NiH4, showed decreased activity toward the hydrogenation of ethylene, representing the direct opposite behavior to that for RENi5–xAlx alloys. We considered the reason for decline in catalytic activity of Mg2NiH4 from the electronic states. The reason can be the low ability to break multiple bonds in hydrocarbons or single bonds in hydrogen molecules due to a lack of an electron supply from the non-metallic Mg2NiH4.
中文翻译:

RENi5–xAlx (RE = La, Er) 的高催化活性和 Mg2Ni 在氢吸收后的低活性:吸收氢的作用
金属间化合物 (IMC) 的催化作用最近受到了极大的关注,并且还能够储存氢的 IMC,包括 RENi 5– x Al x (RE = La, Er) 和 Mg 2 Ni,已被用作加氢催化剂乙烯。吸收氢的RENi 5– x Al x表现出更高的催化活性和高水平的乙烯转化率。RENi 5– x Al x的反应速率吸收的氢高于纯镍和铜,接近钯。脉冲气体测试表明,吸收氢的存在对于高催化活性是必不可少的。在这项工作中,通过使用氘进行试验来评估吸收的氢在催化反应中的作用。发现在 ErNi 3.75 Al 1.25 D n中吸收的氘与乙烯几乎没有反应。一种不同于吸收氢直接与C 2 H 4反应的新型活化机制被提议。基于吸附实验和电子态的密度泛函理论计算研究了这种机制。结论是,弱吸附的乙烯和氢在氢化物表面比在没有吸附氢的基体表面更具反应性,导致催化活性增加。相比之下,Mg 2 Ni的氢化物Mg 2 NiH 4对乙烯的加氢活性降低,这与 RENi 5- x Al x合金的行为直接相反。我们考虑了Mg 2 NiH 4催化活性下降的原因从电子状态。原因可能是由于缺乏来自非金属 Mg 2 NiH 4的电子供应,因此破坏碳氢化合物中的多键或氢分子中的单键的能力较低。
更新日期:2021-09-30
中文翻译:

RENi5–xAlx (RE = La, Er) 的高催化活性和 Mg2Ni 在氢吸收后的低活性:吸收氢的作用
金属间化合物 (IMC) 的催化作用最近受到了极大的关注,并且还能够储存氢的 IMC,包括 RENi 5– x Al x (RE = La, Er) 和 Mg 2 Ni,已被用作加氢催化剂乙烯。吸收氢的RENi 5– x Al x表现出更高的催化活性和高水平的乙烯转化率。RENi 5– x Al x的反应速率吸收的氢高于纯镍和铜,接近钯。脉冲气体测试表明,吸收氢的存在对于高催化活性是必不可少的。在这项工作中,通过使用氘进行试验来评估吸收的氢在催化反应中的作用。发现在 ErNi 3.75 Al 1.25 D n中吸收的氘与乙烯几乎没有反应。一种不同于吸收氢直接与C 2 H 4反应的新型活化机制被提议。基于吸附实验和电子态的密度泛函理论计算研究了这种机制。结论是,弱吸附的乙烯和氢在氢化物表面比在没有吸附氢的基体表面更具反应性,导致催化活性增加。相比之下,Mg 2 Ni的氢化物Mg 2 NiH 4对乙烯的加氢活性降低,这与 RENi 5- x Al x合金的行为直接相反。我们考虑了Mg 2 NiH 4催化活性下降的原因从电子状态。原因可能是由于缺乏来自非金属 Mg 2 NiH 4的电子供应,因此破坏碳氢化合物中的多键或氢分子中的单键的能力较低。



























 京公网安备 11010802027423号
京公网安备 11010802027423号