当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Construction of Enantioenriched γ,γ-Disubstituted Butenolides Enabled by Chiral Amine and Lewis Acid Cascade Cocatalysis
Organic Letters ( IF 5.2 ) Pub Date : 2021-09-20 , DOI: 10.1021/acs.orglett.1c02916 Chenguang Yu 1, 2 , Peng Ji 1 , Yueteng Zhang 1 , Xiang Meng 1 , Wei Wang 1
Organic Letters ( IF 5.2 ) Pub Date : 2021-09-20 , DOI: 10.1021/acs.orglett.1c02916 Chenguang Yu 1, 2 , Peng Ji 1 , Yueteng Zhang 1 , Xiang Meng 1 , Wei Wang 1
Affiliation
|
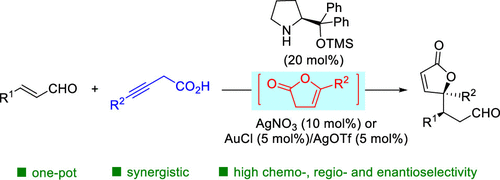
Herein we report a cascade cocatalysis strategy for the facile construction of chiral γ,γ-disubstituted butenolides. The synthetic manifold employs simple alkynoic acids instead of the preformed silyloxy furans or 5-substituted furan-2(3H)-ones. In situ formed 5-substituted furan-2(3H)-ones by AgNO3 or Ph3PAuCl/AgOTf catalyzed cyclization of alkynoic acids can smoothly engage in the subsequent chiral diphenylprolinol TMS-ether catalyzed Michael and Michael-aldol reactions. The cascade process serves as a general approach to chiral quaternary γ,γ-disubstituted butenolides.
中文翻译:

通过手性胺和路易斯酸级联共催化构建对映体富集的 γ,γ-二取代丁烯内酯
在此,我们报告了一种用于轻松构建手性 γ,γ-二取代丁烯内酯的级联共催化策略。合成歧管使用简单的炔酸代替预先形成的甲硅烷氧基呋喃或 5-取代呋喃-2(3 H )-酮。通过AgNO 3或Ph 3 PAuCl/AgOTf 催化的炔酸环化原位形成的5-取代呋喃-2(3 H )-酮可以顺利地参与随后的手性二苯基脯氨醇TMS-醚催化的迈克尔和迈克尔-羟醛反应。级联过程是手性季 γ,γ-二取代丁烯内酯的通用方法。
更新日期:2021-10-01
中文翻译:

通过手性胺和路易斯酸级联共催化构建对映体富集的 γ,γ-二取代丁烯内酯
在此,我们报告了一种用于轻松构建手性 γ,γ-二取代丁烯内酯的级联共催化策略。合成歧管使用简单的炔酸代替预先形成的甲硅烷氧基呋喃或 5-取代呋喃-2(3 H )-酮。通过AgNO 3或Ph 3 PAuCl/AgOTf 催化的炔酸环化原位形成的5-取代呋喃-2(3 H )-酮可以顺利地参与随后的手性二苯基脯氨醇TMS-醚催化的迈克尔和迈克尔-羟醛反应。级联过程是手性季 γ,γ-二取代丁烯内酯的通用方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号