当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Sc(OTf)3-Catalyzed C2-Selective Cyanation/Defluorination Cascade of Perfluoroalkylated 3-Indolylmethanols and Application to the Synthesis of 3-Fluoro(perfluoroalkyl)-β-carbolines
Organic Letters ( IF 5.2 ) Pub Date : 2021-09-20 , DOI: 10.1021/acs.orglett.1c02932 Jingjing Sang 1 , Li Feng 1 , Rui Hu 1 , Jichao Chen 1 , Dandan Shang 1 , Qing Bao 1 , Weidong Rao 1
Organic Letters ( IF 5.2 ) Pub Date : 2021-09-20 , DOI: 10.1021/acs.orglett.1c02932 Jingjing Sang 1 , Li Feng 1 , Rui Hu 1 , Jichao Chen 1 , Dandan Shang 1 , Qing Bao 1 , Weidong Rao 1
Affiliation
|
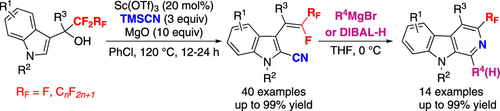
An unprecedented Sc(OTf)3-catalyzed C2-selective cyanation/defluorination cascade of perfluoroalkylated 3-indolylmethanols with TMSCN is described, which provides a novel and practical strategy for the synthesis of structurally diverse 3-(2-cyano)-indolyl substituted gem-difluoroalkenes and β-fluoro-β-perfluoroalkylalkenes. The reaction features excellent regio- and stereoselectivity and broad substrate scope. Notably, the obtained gem-difluoroalkenes and β-fluoro-β-perfluoroalkylalkenes could be easily transformed into 3-fluoro(perfluoroalkyl)-β-carbolines with excellent efficiency simply by treating them with Grignard reagents or DIBAL-H under mild reaction conditions.
中文翻译:

Sc(OTf)3-催化全氟烷基化 3-吲哚甲醇的 C2-选择性氰化/脱氟级联及其在 3-氟(全氟烷基)-β-咔啉的合成中的应用
描述了一种前所未有的 Sc(OTf) 3催化 C2 选择性氰化/脱氟级联的全氟烷基化 3-吲哚甲醇与 TMSCN,这为合成结构多样的 3-(2-氰基)-吲哚基取代的宝石提供了一种新颖实用的策略-二氟烯烃和β-氟-β-全氟烷基烯烃。该反应具有优异的区域和立体选择性和广泛的底物范围。值得注意的是,得到的钆-二氟烯烃和β-氟-β-全氟烷基烯烃可以很容易地转化为3-氟(全氟烷基)-β-咔啉,只需在温和的反应条件下用格氏试剂或DIBAL-H处理即可。
更新日期:2021-10-01
中文翻译:

Sc(OTf)3-催化全氟烷基化 3-吲哚甲醇的 C2-选择性氰化/脱氟级联及其在 3-氟(全氟烷基)-β-咔啉的合成中的应用
描述了一种前所未有的 Sc(OTf) 3催化 C2 选择性氰化/脱氟级联的全氟烷基化 3-吲哚甲醇与 TMSCN,这为合成结构多样的 3-(2-氰基)-吲哚基取代的宝石提供了一种新颖实用的策略-二氟烯烃和β-氟-β-全氟烷基烯烃。该反应具有优异的区域和立体选择性和广泛的底物范围。值得注意的是,得到的钆-二氟烯烃和β-氟-β-全氟烷基烯烃可以很容易地转化为3-氟(全氟烷基)-β-咔啉,只需在温和的反应条件下用格氏试剂或DIBAL-H处理即可。


























 京公网安备 11010802027423号
京公网安备 11010802027423号