当前位置:
X-MOL 学术
›
Environ. Sci.: Nano
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Unravelling the facets-dependent behavior among H2O2, O3 and oxygen vacancies on CeOx and the promotion of peroxone reaction at under acidic conditions
Environmental Science: Nano ( IF 7.3 ) Pub Date : 2021-09-10 , DOI: 10.1039/d1en00716e Weirui Chen 1, 2, 3, 4 , Hengxi He 1, 2, 3, 4 , Ruini Zou 1 , Yidan Chen 1 , Xukai Li 1, 2, 3, 4 , Jing Wang 1, 2, 3, 4 , Yiming Tang 1, 2, 3, 4 , Laisheng Li 1, 2, 3, 4
Environmental Science: Nano ( IF 7.3 ) Pub Date : 2021-09-10 , DOI: 10.1039/d1en00716e Weirui Chen 1, 2, 3, 4 , Hengxi He 1, 2, 3, 4 , Ruini Zou 1 , Yidan Chen 1 , Xukai Li 1, 2, 3, 4 , Jing Wang 1, 2, 3, 4 , Yiming Tang 1, 2, 3, 4 , Laisheng Li 1, 2, 3, 4
Affiliation
|
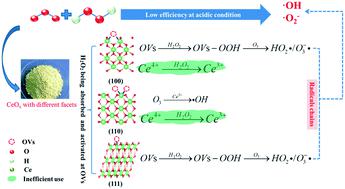
In this study, CeOx with different exposed facets was prepared to investigate their potential effect on activating the peroxone reaction (H2O2/O3) and breaking through the restriction of solution pH on peroxone. By controlling the concentration of hydroxide, cube CeOx with a (100) facet, rod CeOx with a (110) facet and octahedron CeOx with a (111) facet were prepared by the hydrothermal precipitation method, and they all promoted heterogeneous peroxone for oxalic acid (OA) under acidic conditions. The catalytic activity of CeOx was highly related to their surface oxygen vacancies (OVs) and the interfacial electron transfer process. Both experiments and theoretical calculations showed that CeOx with different exposed facets activated peroxone in different ways and (111) CeOx possessed better activity than (100) or (110) CeOx. The low efficiencies of (100) and (110) CeOx were due to their inefficient use of H2O2 to reduce surface Ce4+ and their large energy gap to generate surface peroxide (OVs-OOH). (111) CeOx was stable with surface-adsorbed H2O2, and the rich electrons at OVs could be donated to H2O2 and generate OVs-OOH, which then triggered O3 decomposition into hydroxyl radical (˙OH) and superoxide radical (˙O2−). These electron migration processes depended on the electron-donating ability of the OVs rather than solution pH, therefore, still greater amounts of reactive species were generated under acidic conditions. The flexibility of the (111) CeOx/H2O2/O3 process was further investigated for removing 20 kinds of pharmaceuticals. The synergy effect of (111) CeOx/O3 and H2O2 varied with the structure of the pharmaceuticals.
中文翻译:

揭示 CeOx 上 H2O2、O3 和氧空位之间的多面依赖性行为以及酸性条件下过氧酮反应的促进
在本研究中,制备了具有不同暴露面的CeO x以研究它们对激活过氧酮反应 (H 2 O 2 /O 3 ) 和突破溶液 pH 对过氧酮的限制的潜在影响。通过控制氢氧化物的浓度,水热沉淀法制备了具有(100)面的立方CeO x、具有(110)面的棒状CeO x和具有(111)面的八面体CeO x,它们均促进了异质过氧酮用于酸性条件下的草酸 (OA)。CeO x的催化活性与它们的表面氧空位(OV)和界面电子转移过程高度相关。实验和理论计算均表明,具有不同暴露面的CeO x以不同方式激活过氧酮,并且(111)CeO x比(100)或(110)CeO x具有更好的活性。(100) 和(110) CeO x的低效率是由于它们低效地使用H 2 O 2来还原表面Ce 4+以及它们产生表面过氧化物(OVs-OOH)的大能隙。(111) CeO x与表面吸附的 H 2 O 2稳定,OVs 处的富电子可以捐赠给 H2 O 2并生成OVs-OOH,然后触发O 3分解为羟基自由基(˙OH)和超氧自由基(˙O 2 − )。这些电子迁移过程取决于 OV 的给电子能力,而不是溶液的 pH 值,因此,在酸性条件下会产生更多的活性物质。进一步研究了(111)CeO x /H 2 O 2 /O 3工艺去除20种药物的灵活性。(111) CeO x /O 3与H 2 O 2的协同效应 因药物结构而异。
更新日期:2021-09-21
中文翻译:

揭示 CeOx 上 H2O2、O3 和氧空位之间的多面依赖性行为以及酸性条件下过氧酮反应的促进
在本研究中,制备了具有不同暴露面的CeO x以研究它们对激活过氧酮反应 (H 2 O 2 /O 3 ) 和突破溶液 pH 对过氧酮的限制的潜在影响。通过控制氢氧化物的浓度,水热沉淀法制备了具有(100)面的立方CeO x、具有(110)面的棒状CeO x和具有(111)面的八面体CeO x,它们均促进了异质过氧酮用于酸性条件下的草酸 (OA)。CeO x的催化活性与它们的表面氧空位(OV)和界面电子转移过程高度相关。实验和理论计算均表明,具有不同暴露面的CeO x以不同方式激活过氧酮,并且(111)CeO x比(100)或(110)CeO x具有更好的活性。(100) 和(110) CeO x的低效率是由于它们低效地使用H 2 O 2来还原表面Ce 4+以及它们产生表面过氧化物(OVs-OOH)的大能隙。(111) CeO x与表面吸附的 H 2 O 2稳定,OVs 处的富电子可以捐赠给 H2 O 2并生成OVs-OOH,然后触发O 3分解为羟基自由基(˙OH)和超氧自由基(˙O 2 − )。这些电子迁移过程取决于 OV 的给电子能力,而不是溶液的 pH 值,因此,在酸性条件下会产生更多的活性物质。进一步研究了(111)CeO x /H 2 O 2 /O 3工艺去除20种药物的灵活性。(111) CeO x /O 3与H 2 O 2的协同效应 因药物结构而异。

























 京公网安备 11010802027423号
京公网安备 11010802027423号