当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Photoinduced Covalent Irreversible Inactivation of Proline Dehydrogenase by S-Heterocycles
ACS Chemical Biology ( IF 4 ) Pub Date : 2021-09-20 , DOI: 10.1021/acschembio.1c00427 Ashley C Campbell 1 , Austin R Prater 1 , Alexandra N Bogner 1 , Thomas P Quinn 1 , Kent S Gates 2 , Donald F Becker 3 , John J Tanner 1, 2
ACS Chemical Biology ( IF 4 ) Pub Date : 2021-09-20 , DOI: 10.1021/acschembio.1c00427 Ashley C Campbell 1 , Austin R Prater 1 , Alexandra N Bogner 1 , Thomas P Quinn 1 , Kent S Gates 2 , Donald F Becker 3 , John J Tanner 1, 2
Affiliation
|
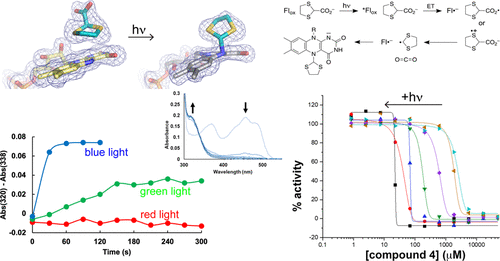
Proline dehydrogenase (PRODH) is a flavoenzyme that catalyzes the first step of proline catabolism, the oxidation of l-proline to Δ1-pyrroline-5-carboxylate. PRODH has emerged as a cancer therapy target because of its involvement in the metabolic reprogramming of cancer cells. Here, we report the discovery of a new class of PRODH inactivator, which covalently and irreversibly modifies the FAD in a light-dependent manner. Two examples, 1,3-dithiolane-2-carboxylate and tetrahydrothiophene-2-carboxylate, have been characterized using X-ray crystallography (1.52–1.85 Å resolution), absorbance spectroscopy, and enzyme kinetics. The structures reveal that in the dark, these compounds function as classical reversible, proline analogue inhibitors. However, exposure of enzyme-inhibitor cocrystals to bright white light induces decarboxylation of the inhibitor and covalent attachment of the residual S-heterocycle to the FAD N5 atom, locking the cofactor into a reduced, inactive state. Spectroscopic measurements of the inactivation process in solution confirm the requirement for light and show that blue light is preferred. Enzyme activity assays show that the rate of inactivation is enhanced by light and that the inactivation is irreversible. We also demonstrate the photosensitivity of cancer cells to one of these compounds. A possible mechanism is proposed involving photoexcitation of the FAD, while the inhibitor is noncovalently bound in the active site, followed by electron transfer, decarboxylation, and radical combination steps. Our results could lead to the development of photopharmacological drugs targeting PRODH.
中文翻译:

S-杂环化合物对脯氨酸脱氢酶的光诱导共价不可逆失活
脯氨酸脱氢酶 (PRODH) 是一种黄素酶,可催化脯氨酸分解代谢的第一步,将l -脯氨酸氧化为 Δ 1-吡咯啉-5-羧酸盐。PRODH 已成为癌症治疗靶点,因为它参与癌细胞的代谢重编程。在这里,我们报告了一类新的 PRODH 灭活剂的发现,它以光依赖的方式共价且不可逆地修饰 FAD。两个例子,1,3-dithiolane-2-carboxylate 和 tetrahydrothiophene-2-carboxylate,已经使用 X 射线晶体学(1.52–1.85 Å 分辨率)、吸收光谱和酶动力学进行了表征。这些结构表明,在黑暗中,这些化合物起到经典的可逆脯氨酸类似物抑制剂的作用。然而,酶抑制剂共晶体暴露在明亮的白光下会诱导抑制剂脱羧,并将残留的 S-杂环共价连接到 FAD N5 原子上,从而将辅助因子锁定在还原的、无活性的状态。溶液中灭活过程的光谱测量证实了对光的需求,并表明蓝光是首选。酶活性测定显示失活率因光而增强,并且失活是不可逆的。我们还证明了癌细胞对其中一种化合物的光敏性。提出了一种可能的机制,涉及 FAD 的光激发,而抑制剂非共价结合在活性位点,然后是电子转移、脱羧和自由基结合步骤。我们的结果可能会导致针对 PRODH 的光药理学药物的开发。酶活性测定显示失活率因光而增强,并且失活是不可逆的。我们还证明了癌细胞对其中一种化合物的光敏性。提出了一种可能的机制,涉及 FAD 的光激发,而抑制剂非共价结合在活性位点,然后是电子转移、脱羧和自由基结合步骤。我们的结果可能会导致针对 PRODH 的光药理学药物的开发。酶活性测定显示失活率因光而增强,并且失活是不可逆的。我们还证明了癌细胞对其中一种化合物的光敏性。提出了一种可能的机制,涉及 FAD 的光激发,而抑制剂非共价结合在活性位点,然后是电子转移、脱羧和自由基结合步骤。我们的结果可能会导致针对 PRODH 的光药理学药物的开发。
更新日期:2021-11-19
中文翻译:

S-杂环化合物对脯氨酸脱氢酶的光诱导共价不可逆失活
脯氨酸脱氢酶 (PRODH) 是一种黄素酶,可催化脯氨酸分解代谢的第一步,将l -脯氨酸氧化为 Δ 1-吡咯啉-5-羧酸盐。PRODH 已成为癌症治疗靶点,因为它参与癌细胞的代谢重编程。在这里,我们报告了一类新的 PRODH 灭活剂的发现,它以光依赖的方式共价且不可逆地修饰 FAD。两个例子,1,3-dithiolane-2-carboxylate 和 tetrahydrothiophene-2-carboxylate,已经使用 X 射线晶体学(1.52–1.85 Å 分辨率)、吸收光谱和酶动力学进行了表征。这些结构表明,在黑暗中,这些化合物起到经典的可逆脯氨酸类似物抑制剂的作用。然而,酶抑制剂共晶体暴露在明亮的白光下会诱导抑制剂脱羧,并将残留的 S-杂环共价连接到 FAD N5 原子上,从而将辅助因子锁定在还原的、无活性的状态。溶液中灭活过程的光谱测量证实了对光的需求,并表明蓝光是首选。酶活性测定显示失活率因光而增强,并且失活是不可逆的。我们还证明了癌细胞对其中一种化合物的光敏性。提出了一种可能的机制,涉及 FAD 的光激发,而抑制剂非共价结合在活性位点,然后是电子转移、脱羧和自由基结合步骤。我们的结果可能会导致针对 PRODH 的光药理学药物的开发。酶活性测定显示失活率因光而增强,并且失活是不可逆的。我们还证明了癌细胞对其中一种化合物的光敏性。提出了一种可能的机制,涉及 FAD 的光激发,而抑制剂非共价结合在活性位点,然后是电子转移、脱羧和自由基结合步骤。我们的结果可能会导致针对 PRODH 的光药理学药物的开发。酶活性测定显示失活率因光而增强,并且失活是不可逆的。我们还证明了癌细胞对其中一种化合物的光敏性。提出了一种可能的机制,涉及 FAD 的光激发,而抑制剂非共价结合在活性位点,然后是电子转移、脱羧和自由基结合步骤。我们的结果可能会导致针对 PRODH 的光药理学药物的开发。

























 京公网安备 11010802027423号
京公网安备 11010802027423号