Molecular Catalysis ( IF 4.6 ) Pub Date : 2021-09-20 , DOI: 10.1016/j.mcat.2021.111871 Megha 1, 2 , Arup Banerjee 1, 2 , Tapan K. Ghanty 2, 3
|
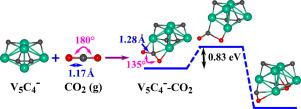
Adsorption and activation of onto small-sized transition metal clusters is of practical importance for the conversion of this molecule to value-added products. We study the efficacy of small anionic vanadium carbide clusters, with n = 1–6 towards the aforementioned key step by employing density functional theory based method. One of such clusters has been shown to perform very well in activating molecule as revealed in a recent experiment. The results of our calculations suggest that the binding of molecule with the anionic vanadium carbide clusters becomes stronger with increase in number of vanadium atoms in the cluster. This adsorption energy trend can be properly rationalized by using the d-band center model. The strong adsorption of molecule is also accompanied with its high degree of activation. The high activation of is characterized by a significant amount of charge transfer from the anionic clusters to the molecule. We further find that the activation barrier heights for the dissociation of to CO and O fragments are considerably small for and clusters. In particular, cluster with an activation barrier of 0.83 eV may serve as a good candidate for activation and dissociation of a molecule.
中文翻译:

CO2 分子在亚纳米级阴离子碳化钒簇 VnC4− (n = 1–6) 上的吸附和活化:理论研究
吸附和活化 转移到小尺寸过渡金属簇上对于将该分子转化为增值产品具有实际重要性。我们研究了小的阴离子碳化钒簇的功效,通过采用基于密度泛函理论的方法,n = 1-6 朝着上述关键步骤迈进。其中一个集群已被证明在激活在最近的实验中揭示的分子。我们的计算结果表明,结合具有阴离子碳化钒簇的分子随着簇中钒原子数的增加而变得更强。这种吸附能趋势可以通过使用 d 带中心模型适当地合理化。强吸附性分子还伴随着它的高度活化。高活性其特征在于大量电荷从阴离子簇转移到分子。我们进一步发现,解离的激活势垒高度 CO 和 O 碎片相当小 和 集群。特别是, 具有 0.83 eV 激活势垒的簇可以作为激活和解离的一个很好的候选者 分子。



























 京公网安备 11010802027423号
京公网安备 11010802027423号