Materials Today Physics ( IF 11.5 ) Pub Date : 2021-09-20 , DOI: 10.1016/j.mtphys.2021.100539 Sainan Zhou 1 , Maohuai Wang 2 , Shuxian Wei 3 , Huili Xin 3 , Wanru Zhai 1 , Shengyu Xu 1 , Sen Liu 3 , Siyuan Liu 1 , Zhaojie Wang 1 , Chi-Man Lawrence Wu 2 , Xiaoqing Lu 1
|
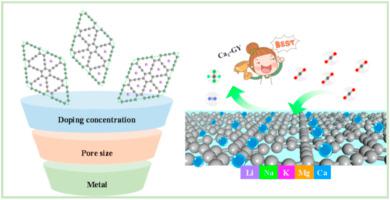
Adsorbents with excellent CO2 capture and separation performances are critical in reducing the excessive CO2 concentration in the atmosphere. Herein, alkali/alkaline earth metals doped graphynes (AMn-GYs, AM = Li, Na, K, Mg and Ca; GY = graphyne; n = 0.5, 1, and 2) were evaluated by employing grand canonical Monte Carlo and density functional theory approaches. The results showed that AMs were strongly bonded on GY with binding energies of −0.09 to −2.52 eV and diffusion barriers of 5.20–16.32 eV. Electronic structure analyses proved that there was a strong covalent bond, large charge transfer, and distinct orbital overlap characters between the AMs and GY, thus constructing a stable and feasible gas adsorption environment. Among all structures, AM1-GYs with one AM doping in a GY unit cell displayed the best CO2 adsorption behavior. Thereafter, Li1/Na1/K1-GY with a pore size of 6.60 Å and Mg1/Ca1-GY with a pore size of 6.50 Å were screened as the potential adsorbents. In comparison, the best performing Ca1-GY had an ultra-high CO2 adsorption capacity of 9.01 mmol/g at 298 K and 1 bar, which was superior to most of the previously reported adsorbents. At 298 K and 1 bar, the CO2 selectivity over N2/CH4 for Ca1-GY reached 2246 and 5602, respectively. Interaction and gas distribution analysis confirmed that comparing to other AM1-GYs, Ca1-GY has a stronger affinity with CO2 and larger isosteric heat differences between CO2 and other gases, rendering Ca1-GY to possess excellent adsorption capacity and selectivity.
中文翻译:

碱/碱土金属掺杂石墨炔的多目标优化用于超高性能 CO2 捕获和分离 N2/CH4
具有出色 CO 2捕获和分离性能的吸附剂对于降低大气中过量的 CO 2浓度至关重要。在此,碱/碱土金属掺杂的石墨烯(AM n -GYs,AM = Li、Na、K、Mg 和 Ca;GY = 石墨炔;n = 0.5、1 和 2)通过使用正则蒙特卡罗和密度进行评估功能理论方法。结果表明,AMs 与 GY 牢固结合,结合能为 -0.09 至 -2.52 eV,扩散势垒为 5.20-16.32 eV。电子结构分析证明,AMs和GY之间存在强共价键、大电荷转移和明显的轨道重叠特征,从而构建了稳定可行的气体吸附环境。在所有结构中,AM在 GY 晶胞中掺杂一种 AM 的1 -GY 表现出最佳的 CO 2吸附行为。此后,筛选出孔径为6.60 Å 的Li 1 /Na 1 /K 1 -GY 和孔径为6.50 Å 的Mg 1 /Ca 1 -GY 作为潜在的吸附剂。相比之下,性能最好的 Ca 1 -GY在 298 K 和 1 bar 下具有 9.01 mmol/g的超高 CO 2吸附容量,优于大多数先前报道的吸附剂。在 298 K 和 1 bar 条件下,Ca 1 相对于 N 2 /CH 4的 CO 2选择性-GY 分别达到 2246 和 5602。相互作用和气体分布分析证实,与其他AM 1 -GY相比,Ca 1 -GY与CO 2 的亲和力更强,CO 2与其他气体的等量热差更大,使Ca 1 -GY具有优异的吸附能力和选择性.



























 京公网安备 11010802027423号
京公网安备 11010802027423号