当前位置:
X-MOL 学术
›
Adv. Healthcare Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Heat-Confined Tumor-Docking Reversible Thermogel Potentiates Systemic Antitumor Immune Response During Near-Infrared Photothermal Ablation in Triple-Negative Breast Cancer
Advanced Healthcare Materials ( IF 10.0 ) Pub Date : 2021-09-20 , DOI: 10.1002/adhm.202100907 Vishnu Revuri 1 , Santhosh Kalash Rajendrakumar 2 , Myong-Suk Park 3 , Adityanarayan Mohapatra 2 , Saji Uthaman 2 , Jagannath Mondal 1 , Woo Kyun Bae 3 , In-Kyu Park 2 , Yong-Kyu Lee 1
Advanced Healthcare Materials ( IF 10.0 ) Pub Date : 2021-09-20 , DOI: 10.1002/adhm.202100907 Vishnu Revuri 1 , Santhosh Kalash Rajendrakumar 2 , Myong-Suk Park 3 , Adityanarayan Mohapatra 2 , Saji Uthaman 2 , Jagannath Mondal 1 , Woo Kyun Bae 3 , In-Kyu Park 2 , Yong-Kyu Lee 1
Affiliation
|
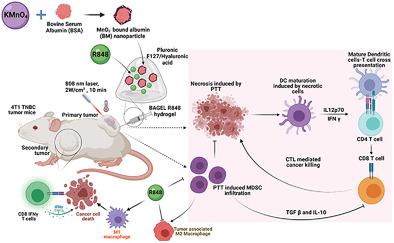
Triple-negative breast cancer (TNBC) features immunologically “cold” tumor microenvironments with limited cytotoxic T lymphocyte (CTL) infiltration. Although ablation therapies have demonstrated modulation of “cold” TNBC tumors to inflamed “hot” tumors, recruitment of myeloid derived suppressor cells (MDSCs) at the tumors post ablation therapies prevents the infiltration of CTLs and challenge the antitumor potentials of T-cell therapies. Here, a thermal ablation immunotherapy strategy is developed to prevent the immune suppressive effects of MDSCs during photothermal ablation and induce a durable systemic antitumor immunity to eradicate TNBC tumors. An injectable pluronic F127/hyaluronic acid (HA)-based hydrogel embedded with manganese dioxide (BM) nanoparticles and TLR7 agonist resiquimod (R848) (BAGEL-R848), is synthesized to induce in situ laser-assisted gelation of the hydrogel and achieve desired ablation temperatures at a low laser-exposure time. Upon 808-nm laser irradiation, a significant reduction in the tumor burden is observed in BAGEL-R848-injected 4T1 tumor-bearing mice. The ablation induced immunogenic cell death and sustained release of R848 from BAGEL-R848 promotes dendritic cell maturation and reduced MDSCs localization in tumors. In addition, inflammatory M1 macrophages and CD8+IFN+ CTL are enriched in distant tumors in bilateral 4T1 tumor model, preventing metastatic tumor growth and signifying the potential of BAGEL-R848 to treat TNBC.
中文翻译:

热限制性肿瘤对接可逆热凝胶在三阴性乳腺癌的近红外光热消融过程中增强全身抗肿瘤免疫反应
三阴性乳腺癌 (TNBC) 具有免疫“冷”肿瘤微环境,细胞毒性 T 淋巴细胞 (CTL) 浸润有限。尽管消融疗法已证明“冷”TNBC 肿瘤可调节为发炎的“热”肿瘤,但在消融治疗后在肿瘤中募集骨髓源性抑制细胞 (MDSC) 可防止 CTL 浸润并挑战 T 细胞疗法的抗肿瘤潜力。在这里,开发了一种热消融免疫治疗策略,以防止 MDSCs 在光热消融过程中的免疫抑制作用,并诱导持久的全身抗肿瘤免疫以根除 TNBC 肿瘤。一种可注射的 pluronic F127/透明质酸 (HA) 基水凝胶,嵌入了二氧化锰 (BM) 纳米颗粒和 TLR7 激动剂瑞喹莫特 (R848) (BAGEL-R848),合成以诱导水凝胶的原位激光辅助凝胶化,并在低激光曝光时间下达到所需的消融温度。在 808 nm 激光照射下,在注射 BAGEL-R848 的 4T1 荷瘤小鼠中观察到肿瘤负荷显着减少。消融诱导的免疫原性细胞死亡和 R848 从 BAGEL-R848 的持续释放促进树突细胞成熟并减少 MDSC 在肿瘤中的定位。此外,炎性 M1 巨噬细胞和 CD8+IFN+CTL 在双侧 4T1 肿瘤模型的远处肿瘤中富集,防止转移性肿瘤生长并表明 BAGEL-R848 治疗 TNBC 的潜力。在注射 BAGEL-R848 的 4T1 荷瘤小鼠中观察到肿瘤负荷显着降低。消融诱导的免疫原性细胞死亡和 R848 从 BAGEL-R848 的持续释放促进树突细胞成熟并减少 MDSC 在肿瘤中的定位。此外,炎性 M1 巨噬细胞和 CD8+IFN+CTL 在双侧 4T1 肿瘤模型的远处肿瘤中富集,防止转移性肿瘤生长并表明 BAGEL-R848 治疗 TNBC 的潜力。在注射 BAGEL-R848 的 4T1 荷瘤小鼠中观察到肿瘤负荷显着降低。消融诱导的免疫原性细胞死亡和 R848 从 BAGEL-R848 的持续释放促进树突细胞成熟并减少 MDSC 在肿瘤中的定位。此外,炎性 M1 巨噬细胞和 CD8+IFN+CTL 在双侧 4T1 肿瘤模型的远处肿瘤中富集,防止转移性肿瘤生长并表明 BAGEL-R848 治疗 TNBC 的潜力。
更新日期:2021-11-04
中文翻译:

热限制性肿瘤对接可逆热凝胶在三阴性乳腺癌的近红外光热消融过程中增强全身抗肿瘤免疫反应
三阴性乳腺癌 (TNBC) 具有免疫“冷”肿瘤微环境,细胞毒性 T 淋巴细胞 (CTL) 浸润有限。尽管消融疗法已证明“冷”TNBC 肿瘤可调节为发炎的“热”肿瘤,但在消融治疗后在肿瘤中募集骨髓源性抑制细胞 (MDSC) 可防止 CTL 浸润并挑战 T 细胞疗法的抗肿瘤潜力。在这里,开发了一种热消融免疫治疗策略,以防止 MDSCs 在光热消融过程中的免疫抑制作用,并诱导持久的全身抗肿瘤免疫以根除 TNBC 肿瘤。一种可注射的 pluronic F127/透明质酸 (HA) 基水凝胶,嵌入了二氧化锰 (BM) 纳米颗粒和 TLR7 激动剂瑞喹莫特 (R848) (BAGEL-R848),合成以诱导水凝胶的原位激光辅助凝胶化,并在低激光曝光时间下达到所需的消融温度。在 808 nm 激光照射下,在注射 BAGEL-R848 的 4T1 荷瘤小鼠中观察到肿瘤负荷显着减少。消融诱导的免疫原性细胞死亡和 R848 从 BAGEL-R848 的持续释放促进树突细胞成熟并减少 MDSC 在肿瘤中的定位。此外,炎性 M1 巨噬细胞和 CD8+IFN+CTL 在双侧 4T1 肿瘤模型的远处肿瘤中富集,防止转移性肿瘤生长并表明 BAGEL-R848 治疗 TNBC 的潜力。在注射 BAGEL-R848 的 4T1 荷瘤小鼠中观察到肿瘤负荷显着降低。消融诱导的免疫原性细胞死亡和 R848 从 BAGEL-R848 的持续释放促进树突细胞成熟并减少 MDSC 在肿瘤中的定位。此外,炎性 M1 巨噬细胞和 CD8+IFN+CTL 在双侧 4T1 肿瘤模型的远处肿瘤中富集,防止转移性肿瘤生长并表明 BAGEL-R848 治疗 TNBC 的潜力。在注射 BAGEL-R848 的 4T1 荷瘤小鼠中观察到肿瘤负荷显着降低。消融诱导的免疫原性细胞死亡和 R848 从 BAGEL-R848 的持续释放促进树突细胞成熟并减少 MDSC 在肿瘤中的定位。此外,炎性 M1 巨噬细胞和 CD8+IFN+CTL 在双侧 4T1 肿瘤模型的远处肿瘤中富集,防止转移性肿瘤生长并表明 BAGEL-R848 治疗 TNBC 的潜力。


























 京公网安备 11010802027423号
京公网安备 11010802027423号