当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Hydrothermal One-Electron Oxidation of Carboxylic Acids in the Presence of Iron Oxide Minerals
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2021-09-17 , DOI: 10.1021/acsearthspacechem.1c00139 Kristin N. Johnson-Finn 1, 2 , Lynda B. Williams 3 , Ian R. Gould 2 , Hilairy E. Hartnett 2, 3 , Everett L. Shock 2, 3
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2021-09-17 , DOI: 10.1021/acsearthspacechem.1c00139 Kristin N. Johnson-Finn 1, 2 , Lynda B. Williams 3 , Ian R. Gould 2 , Hilairy E. Hartnett 2, 3 , Everett L. Shock 2, 3
Affiliation
|
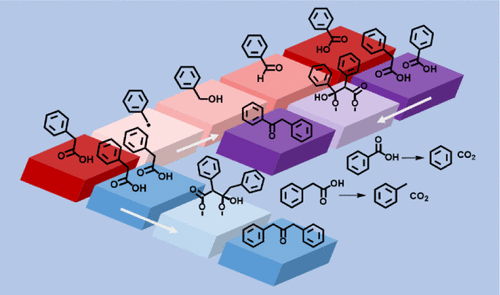
Hydrothermal experiments were undertaken to explore the reaction pathways of phenyl-containing carboxylic acids in the presence of iron oxide minerals. At 300 °C and 1 kbar (100 MPa), in addition to the previously reported decarboxylation and ketonic decarboxylation pathways, phenylacetic acid undergoes oxidation to form benzoic acid, which eventually forms 2-phenylacetophenone via ketonic decarboxylation with phenylacetic acid. The production of benzoic acid in the presence of magnetite (Fe3O4) or hematite (Fe2O3) parallels the production of benzoic acid in the presence of Cu(II) salt solutions observed in previous hydrothermal studies, which was attributed to a sequence of one-electron-transfer processes. We propose a similar one-electron oxidation reaction pathway in the presence of minerals. Complexity builds as the reaction options increase. Hydrothermal experiments with hydrocinnamic acid were performed to demonstrate the generality of the reaction pathways for carboxylic acids, although the rate of consumption of hydrocinnamic acid was slower than that of phenylacetic acid and yielded a complex variety of detected products. Hydrocarbons are produced at the longest observed time points of a reaction through either decomposition or C–C bond formation to larger compounds. These results indicate that minerals can enhance the complexity of organic product pathways for carboxylic acids and resulting products during hydrothermal transformations and may enable the production of hydrocarbons from organic acids and other precursors.
中文翻译:

氧化铁矿物存在下羧酸的水热一电子氧化
进行水热实验以探索含苯基羧酸在氧化铁矿物存在下的反应途径。在 300 °C 和 1 kbar (100 MPa) 条件下,除了之前报道的脱羧和酮脱羧途径外,苯乙酸还经过氧化形成苯甲酸,最终通过与苯乙酸进行酮脱羧形成 2-苯基苯乙酮。在磁铁矿 (Fe 3 O 4 ) 或赤铁矿 (Fe 2 O 3 )存在下生产苯甲酸) 与先前水热研究中观察到的在 Cu(II) 盐溶液存在下苯甲酸的产生相似,这归因于一系列单电子转移过程。我们提出了在矿物质存在下类似的单电子氧化反应途径。复杂性随着反应选项的增加而增加。使用氢化肉桂酸进行水热实验以证明羧酸反应途径的普遍性,尽管氢化肉桂酸的消耗速率比苯乙酸慢,并产生多种复杂的检测产物。碳氢化合物是在观察到的最长反应时间点通过分解或 C-C 键形成更大的化合物产生的。
更新日期:2021-10-22
中文翻译:

氧化铁矿物存在下羧酸的水热一电子氧化
进行水热实验以探索含苯基羧酸在氧化铁矿物存在下的反应途径。在 300 °C 和 1 kbar (100 MPa) 条件下,除了之前报道的脱羧和酮脱羧途径外,苯乙酸还经过氧化形成苯甲酸,最终通过与苯乙酸进行酮脱羧形成 2-苯基苯乙酮。在磁铁矿 (Fe 3 O 4 ) 或赤铁矿 (Fe 2 O 3 )存在下生产苯甲酸) 与先前水热研究中观察到的在 Cu(II) 盐溶液存在下苯甲酸的产生相似,这归因于一系列单电子转移过程。我们提出了在矿物质存在下类似的单电子氧化反应途径。复杂性随着反应选项的增加而增加。使用氢化肉桂酸进行水热实验以证明羧酸反应途径的普遍性,尽管氢化肉桂酸的消耗速率比苯乙酸慢,并产生多种复杂的检测产物。碳氢化合物是在观察到的最长反应时间点通过分解或 C-C 键形成更大的化合物产生的。



























 京公网安备 11010802027423号
京公网安备 11010802027423号