当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Role of Pairwise Reactions on the Synthesis of Li0.3La0.57TiO3 and the Resulting Structure–Property Correlations
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2021-09-17 , DOI: 10.1021/acs.inorgchem.1c02136 Thomas F Malkowski 1 , Robert L Sacci 1 , Rebecca D McAuliffe 1 , Shree Ram Acharya 2 , Valentino R Cooper 2 , Nancy J Dudney 1 , Gabriel M Veith 1
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2021-09-17 , DOI: 10.1021/acs.inorgchem.1c02136 Thomas F Malkowski 1 , Robert L Sacci 1 , Rebecca D McAuliffe 1 , Shree Ram Acharya 2 , Valentino R Cooper 2 , Nancy J Dudney 1 , Gabriel M Veith 1
Affiliation
|
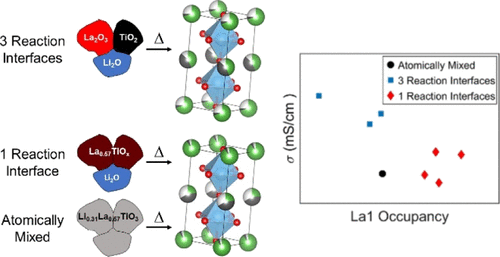
The performance of single-ion conductors is highly sensitive to the material’s defect chemistry. Tuning these defects is limited for solid-state reactions as they occur at particle–particle interfaces, which provide a complex evolving energy landscape for atomic rearrangement and product formation. In this report, we investigate the (1) order of addition and (2) lithium precursor decomposition temperature and their effect on the synthesis and grain boundary conductivity of the perovskite lithium lanthanum titanium oxide (LLTO). We use an intimately mixed sol–gel, a solid-state reaction of Li precursor + La2O3 + TiO2, and Li precursor + amorphous La0.57TiOx as different chemical routes to change the way in which the elements are brought together. The results show that the perovskite can accommodate a wide range of Li deficiencies (upward of 50%) while maintaining the tetragonal LLTO structure, indicating that X-ray diffraction (XRD) is insufficient to fully characterize the chemical nature of the product (i.e., Li-deficient LLTO may behave differently than stoichiometric LLTO). Variations in the relative intensities of different reflections in XRD suggest variations in the La ordering within the crystal structure between synthesis methods. Furthermore, the choice of the precursor and the order of addition of the reactants lower the time required to form a pure phase. Density functional theory calculations of the formation energy of possible reaction intermediates support the hypothesis that a greater thermodynamic driving force to form LLTO leads to a greater LLTO yield. The retention of lithium is correlated with the thermal decomposition temperature of the Li precursor and the starting material mixing strategy. Taking the results together suggests that cations that share a site with Li should be mixed early to avoid ordering. Such cation ordering inhibits Li motion, leading to higher Li ion resistance.
中文翻译:

成对反应在 Li0.3La0.57TiO3 合成中的作用以及由此产生的结构-性能相关性
单离子导体的性能对材料的缺陷化学高度敏感。调整这些缺陷对于固态反应是有限的,因为它们发生在粒子 - 粒子界面,这为原子重排和产物形成提供了复杂的演化能量图谱。在本报告中,我们研究了 (1) 添加顺序和 (2) 锂前驱体分解温度及其对钙钛矿锂镧钛氧化物 (LLTO) 合成和晶界电导率的影响。我们使用紧密混合的溶胶-凝胶,即锂前体 + La 2 O 3 + TiO 2和锂前体 + 无定形 La 0.57 TiO x的固态反应作为改变元素结合方式的不同化学途径。结果表明,钙钛矿可以在保持四方 LLTO 结构的同时适应大范围的锂缺陷(高达 50%),表明 X 射线衍射(XRD)不足以充分表征产物的化学性质(即,缺锂的 LLTO 的行为可能与化学计量的 LLTO 不同)。XRD 中不同反射的相对强度的变化表明合成方法之间晶体结构内 La 排序的变化。此外,前体的选择和反应物的添加顺序降低了形成纯相所需的时间。可能的反应中间体的形成能的密度泛函理论计算支持这样的假设,即形成 LLTO 的更大热力学驱动力导致更大的 LLTO 产率。锂的保留与锂前驱体的热分解温度和起始材料混合策略相关。将结果放在一起表明与 Li 共享一个位点的阳离子应该尽早混合以避免排序。这种阳离子排序抑制锂运动,导致更高的锂离子电阻。将结果放在一起表明与 Li 共享一个位点的阳离子应该尽早混合以避免排序。这种阳离子排序抑制锂运动,导致更高的锂离子电阻。将结果放在一起表明与 Li 共享一个位点的阳离子应该尽早混合以避免排序。这种阳离子排序抑制锂运动,导致更高的锂离子电阻。
更新日期:2021-10-04
中文翻译:

成对反应在 Li0.3La0.57TiO3 合成中的作用以及由此产生的结构-性能相关性
单离子导体的性能对材料的缺陷化学高度敏感。调整这些缺陷对于固态反应是有限的,因为它们发生在粒子 - 粒子界面,这为原子重排和产物形成提供了复杂的演化能量图谱。在本报告中,我们研究了 (1) 添加顺序和 (2) 锂前驱体分解温度及其对钙钛矿锂镧钛氧化物 (LLTO) 合成和晶界电导率的影响。我们使用紧密混合的溶胶-凝胶,即锂前体 + La 2 O 3 + TiO 2和锂前体 + 无定形 La 0.57 TiO x的固态反应作为改变元素结合方式的不同化学途径。结果表明,钙钛矿可以在保持四方 LLTO 结构的同时适应大范围的锂缺陷(高达 50%),表明 X 射线衍射(XRD)不足以充分表征产物的化学性质(即,缺锂的 LLTO 的行为可能与化学计量的 LLTO 不同)。XRD 中不同反射的相对强度的变化表明合成方法之间晶体结构内 La 排序的变化。此外,前体的选择和反应物的添加顺序降低了形成纯相所需的时间。可能的反应中间体的形成能的密度泛函理论计算支持这样的假设,即形成 LLTO 的更大热力学驱动力导致更大的 LLTO 产率。锂的保留与锂前驱体的热分解温度和起始材料混合策略相关。将结果放在一起表明与 Li 共享一个位点的阳离子应该尽早混合以避免排序。这种阳离子排序抑制锂运动,导致更高的锂离子电阻。将结果放在一起表明与 Li 共享一个位点的阳离子应该尽早混合以避免排序。这种阳离子排序抑制锂运动,导致更高的锂离子电阻。将结果放在一起表明与 Li 共享一个位点的阳离子应该尽早混合以避免排序。这种阳离子排序抑制锂运动,导致更高的锂离子电阻。


























 京公网安备 11010802027423号
京公网安备 11010802027423号