当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis and Antimicrobial, Antiproliferative Evaluation of Novel Quinolone and Conazole Analogues via Conventional and Microwave Techniques
ChemistrySelect ( IF 2.1 ) Pub Date : 2021-09-17 , DOI: 10.1002/slct.202101840 Şule Ceylan 1 , Yıldız Uygun Cebeci 2 , Şengül Alpay Karaoğlu 3 , Muhammed Altun 4
ChemistrySelect ( IF 2.1 ) Pub Date : 2021-09-17 , DOI: 10.1002/slct.202101840 Şule Ceylan 1 , Yıldız Uygun Cebeci 2 , Şengül Alpay Karaoğlu 3 , Muhammed Altun 4
Affiliation
|
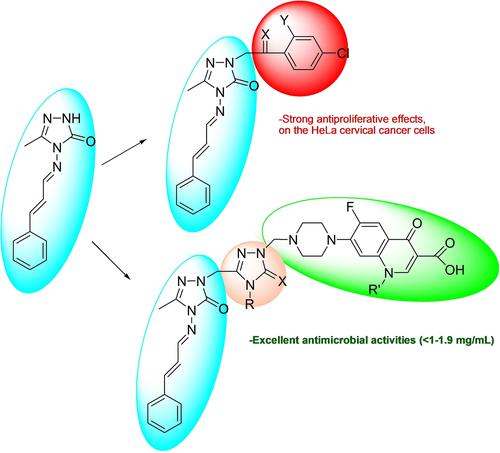
1,2,4-Triazole-3-one (3), acquired from cinnamaldehyde was converted to the corresponding carbox(thio)amides via several steps (6 a–c). Their reaction with sodium hydroxide gave the 1,2,4-triazole derivatives (7 a–c). Compound 3 treatment with 2-bromo-1-(4-chlorophenyl) ethanone or 2-chloro-1-(2,4-dichlorophenyl)ethanone afforded the compounds 8 a,b and by reducing these compounds reduction products were obtained (9 a,b). The synthesis of (10 a–e) was carried out by the reaction compounds 9 a,b with different benzyl chlorides. Then oxadiazole derivative (12) was obtained by ring closure from hydrazide compound 5. Subsequently compounds 3, 7 a–c, and 12 were treated with various amines in the presence of formaldehyde to yield Mannich bases (11 a–e, 14 a–e, 13 a,b). Microwave-assisted and conventional techniques were utilized for the syntheses. The structures of newly synthesized compounds were illuminated by spectroscopic methods. Their antimicrobial (MIC method), and anticancer activities (Abay's method) were examined. Results showed that most of the compounds exhibited good antimicrobial activities. Especially compounds 14 a–e which is a mannich base showed very good antitubercular activity against Mycobacterium smegmatis compared with Streptomycin standard drug. Also compounds 8 a and 9 b have been found to have strong antiproliferative effects on the HeLa cervical cancer cells and also these compounds did not have a cytotoxic effects on a normal cells.
中文翻译:

通过传统和微波技术合成和抗菌、抗增殖评价新型喹诺酮和康唑类似物
从肉桂醛中获得的1,2,4-Triazole-3-one ( 3 ) 通过几个步骤 ( 6 a - c )转化为相应的羧基(硫代)酰胺。他们与氢氧化钠反应得到 1,2,4-三唑衍生物 ( 7 a – c )。化合物3用2-溴-1-(4-氯苯基)乙酮或2-氯-1-(2,4-二氯苯基)乙酮处理得到化合物8a、b,通过还原这些化合物得到还原产物( 9a),乙)。( 10a - e )的合成通过反应化合物9a进行,b与不同的苄基氯。然后由酰肼化合物5通过闭环得到恶二唑衍生物( 12 ) 。随后,化合物3 , 7 a - c和12在甲醛存在下用各种胺处理得到曼尼希碱 ( 11 a - e , 14 a - e , 13 a , b)。微波辅助和常规技术用于合成。通过光谱方法阐明新合成化合物的结构。检查了它们的抗微生物(MIC 方法)和抗癌活性(Abay 方法)。结果表明,大多数化合物表现出良好的抗菌活性。特别是与链霉素标准药物相比,作为曼尼希碱的化合物14a - e对耻垢分枝杆菌显示出非常好的抗结核活性。还发现化合物8a和9b对HeLa宫颈癌细胞具有很强的抗增殖作用,并且这些化合物对正常细胞没有细胞毒性作用。
更新日期:2021-09-17
中文翻译:

通过传统和微波技术合成和抗菌、抗增殖评价新型喹诺酮和康唑类似物
从肉桂醛中获得的1,2,4-Triazole-3-one ( 3 ) 通过几个步骤 ( 6 a - c )转化为相应的羧基(硫代)酰胺。他们与氢氧化钠反应得到 1,2,4-三唑衍生物 ( 7 a – c )。化合物3用2-溴-1-(4-氯苯基)乙酮或2-氯-1-(2,4-二氯苯基)乙酮处理得到化合物8a、b,通过还原这些化合物得到还原产物( 9a),乙)。( 10a - e )的合成通过反应化合物9a进行,b与不同的苄基氯。然后由酰肼化合物5通过闭环得到恶二唑衍生物( 12 ) 。随后,化合物3 , 7 a - c和12在甲醛存在下用各种胺处理得到曼尼希碱 ( 11 a - e , 14 a - e , 13 a , b)。微波辅助和常规技术用于合成。通过光谱方法阐明新合成化合物的结构。检查了它们的抗微生物(MIC 方法)和抗癌活性(Abay 方法)。结果表明,大多数化合物表现出良好的抗菌活性。特别是与链霉素标准药物相比,作为曼尼希碱的化合物14a - e对耻垢分枝杆菌显示出非常好的抗结核活性。还发现化合物8a和9b对HeLa宫颈癌细胞具有很强的抗增殖作用,并且这些化合物对正常细胞没有细胞毒性作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号