当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Stereodivergent Construction of Vicinal Acyclic Quaternary–Tertiary Carbon Stereocenters by Michael-Type Alkylation of α,α-Disubstituted N-tert-Butanesulfinyl Ketimines
Organic Letters ( IF 5.2 ) Pub Date : 2021-09-17 , DOI: 10.1021/acs.orglett.1c02660 Nuermaimaiti Yisimayili 1 , Hui Liu 1 , Yun Yao 2 , Chong-Dao Lu 1, 2
Organic Letters ( IF 5.2 ) Pub Date : 2021-09-17 , DOI: 10.1021/acs.orglett.1c02660 Nuermaimaiti Yisimayili 1 , Hui Liu 1 , Yun Yao 2 , Chong-Dao Lu 1, 2
Affiliation
|
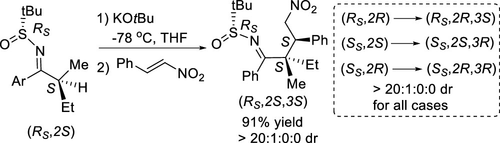
Vicinal quaternary–tertiary carbon stereocenters were constructed with excellent stereoselectivity via aza-enolization of enantioenriched acyclic N-tert-butanesulfinyl ketimines bearing two sterically similar α-linear alkyl substituents followed by conjugate addition to nitroalkenes. Further changes of the absolute configuration of the sulfinyl group and/or the α-stereocenter in the ketimine allowed the facile stereodivergent synthesis of all four diastereomers of the Michael-type alkylation adducts. This reaction is a successful example of acyclic stereocontrol based on stereoselective α-deprotonation for the formation of fully substituted aza-enolates from ketone derivatives.
中文翻译:

通过 α,α-二取代 N-叔丁亚磺酰基酮亚胺的迈克尔型烷基化立体发散构建邻位无环季-叔碳立体中心
连位季叔碳立体物具有优异的立体选择性经由的对映体富集的非环状氮杂-烯醇化构建ñ -叔-butanesulfinyl酮亚胺带有两个空间类似的α-线性烷基取代基,随后共轭加成到硝基烯。酮亚胺中亚磺酰基和/或α-立体中心的绝对构型的进一步变化允许迈克尔型烷基化加合物的所有四种非对映异构体的容易的立体发散合成。该反应是基于立体选择性α-去质子化的无环立体控制的成功例子,用于从酮衍生物形成完全取代的氮杂烯醇化物。
更新日期:2021-10-01
中文翻译:

通过 α,α-二取代 N-叔丁亚磺酰基酮亚胺的迈克尔型烷基化立体发散构建邻位无环季-叔碳立体中心
连位季叔碳立体物具有优异的立体选择性经由的对映体富集的非环状氮杂-烯醇化构建ñ -叔-butanesulfinyl酮亚胺带有两个空间类似的α-线性烷基取代基,随后共轭加成到硝基烯。酮亚胺中亚磺酰基和/或α-立体中心的绝对构型的进一步变化允许迈克尔型烷基化加合物的所有四种非对映异构体的容易的立体发散合成。该反应是基于立体选择性α-去质子化的无环立体控制的成功例子,用于从酮衍生物形成完全取代的氮杂烯醇化物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号