当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
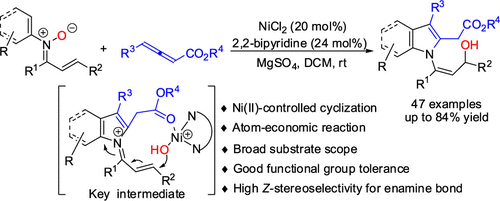
Nickel(II)-Catalyzed [3 + 2] Cycloaddition of Nitrones and Allenoates to Access N-Vinylindoles and N-Vinylpyrroles
Organic Letters ( IF 5.2 ) Pub Date : 2021-09-17 , DOI: 10.1021/acs.orglett.1c02695 Pei-Pei Xu 1 , Jun-Yi Liao 1 , Jia-Jie Zhang 1 , Wei-Min Shi 2 , Cui Liang 1 , Gui-Fa Su 1 , Dong-Liang Mo 1
Organic Letters ( IF 5.2 ) Pub Date : 2021-09-17 , DOI: 10.1021/acs.orglett.1c02695 Pei-Pei Xu 1 , Jun-Yi Liao 1 , Jia-Jie Zhang 1 , Wei-Min Shi 2 , Cui Liang 1 , Gui-Fa Su 1 , Dong-Liang Mo 1
Affiliation
|
A variety of N-vinylindoles and N-vinylpyrroles were prepared in moderate to good yields through the nickel(II)-catalyzed [3 + 2] cycloaddition of α,β-unsaturated nitrones with allenoates under mild reaction conditions. A rational mechanism for the formation of N-vinylindoles was proposed based on the 18O-labeled experiments and key intermediates detected by high-resolution mass spectrometry trace experiments. The present method highlights a nickel(II)-controlled cyclization, atom-economical reaction, broad substrate scope, good functional group tolerance, and high Z-stereoselectivity for the enamine bond.
中文翻译:

镍 (II) 催化 [3 + 2] 环加成硝酮和烯丙酸酯以获取 N-乙烯基吲哚和 N-乙烯基吡咯
在温和的反应条件下,通过镍 (II) 催化的 α,β-不饱和硝酮与烯丙酸酯的 [3 + 2] 环加成反应,以中等至良好的收率制备了多种N-乙烯基吲哚和N-乙烯基吡咯。基于18个O标记实验和高分辨率质谱痕量实验检测到的关键中间体,提出了N-乙烯基吲哚形成的合理机制。本方法突出了镍 (II) 控制的环化、原子经济反应、广泛的底物范围、良好的官能团耐受性和烯胺键的高 Z 立体选择性。
更新日期:2021-10-01
中文翻译:

镍 (II) 催化 [3 + 2] 环加成硝酮和烯丙酸酯以获取 N-乙烯基吲哚和 N-乙烯基吡咯
在温和的反应条件下,通过镍 (II) 催化的 α,β-不饱和硝酮与烯丙酸酯的 [3 + 2] 环加成反应,以中等至良好的收率制备了多种N-乙烯基吲哚和N-乙烯基吡咯。基于18个O标记实验和高分辨率质谱痕量实验检测到的关键中间体,提出了N-乙烯基吲哚形成的合理机制。本方法突出了镍 (II) 控制的环化、原子经济反应、广泛的底物范围、良好的官能团耐受性和烯胺键的高 Z 立体选择性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号