当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesizing Molecules with Linear Tricyclic 5/5/5 and 6/5/5 Skeletons via [5 + 2 + 1]/Ene Strategy
Organic Letters ( IF 5.2 ) Pub Date : 2021-09-17 , DOI: 10.1021/acs.orglett.1c02766 Jing Liu 1 , Yi Zhou 2 , Jiaqi Zhu 2 , Zhi-Xiang Yu 1, 2, 3
Organic Letters ( IF 5.2 ) Pub Date : 2021-09-17 , DOI: 10.1021/acs.orglett.1c02766 Jing Liu 1 , Yi Zhou 2 , Jiaqi Zhu 2 , Zhi-Xiang Yu 1, 2, 3
Affiliation
|
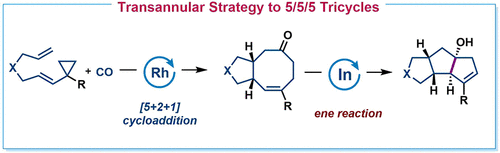
Report here is the development of a [5 + 2 + 1]/ene strategy for the synthesis of molecules with linear tricyclic 5/5/5 and 6/5/5 skeletons widely found in natural products. The first step of this strategy is applying a Rh-catalyzed [5 + 2 + 1] reaction of ene-vinylcyclopropanes and CO to synthesize 5/8 and 6/8 bicyclic compounds, which can then be transformed to the final target molecules by an InCl3-catalyzed ene reaction.
中文翻译:

通过 [5 + 2 + 1]/Ene 策略合成具有线性三环 5/5/5 和 6/5/5 骨架的分子
这里的报告是开发 [5 + 2 + 1]/ene 策略,用于合成具有广泛存在于天然产物中的线性三环 5/5/5 和 6/5/5 骨架的分子。该策略的第一步是应用 Rh 催化的烯-乙烯基环丙烷和 CO 的 [5 + 2 + 1] 反应合成 5/8 和 6/8 双环化合物,然后可以通过以下方式将其转化为最终目标分子InCl 3 -催化的烯反应。
更新日期:2021-10-01
中文翻译:

通过 [5 + 2 + 1]/Ene 策略合成具有线性三环 5/5/5 和 6/5/5 骨架的分子
这里的报告是开发 [5 + 2 + 1]/ene 策略,用于合成具有广泛存在于天然产物中的线性三环 5/5/5 和 6/5/5 骨架的分子。该策略的第一步是应用 Rh 催化的烯-乙烯基环丙烷和 CO 的 [5 + 2 + 1] 反应合成 5/8 和 6/8 双环化合物,然后可以通过以下方式将其转化为最终目标分子InCl 3 -催化的烯反应。



























 京公网安备 11010802027423号
京公网安备 11010802027423号