当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Photoswitching of ortho-Aminated Arylazopyrazoles with Red Light
Organic Letters ( IF 5.2 ) Pub Date : 2021-09-17 , DOI: 10.1021/acs.orglett.1c02856 Julian Simke 1 , Tom Bösking 1 , Bart Jan Ravoo 1
Organic Letters ( IF 5.2 ) Pub Date : 2021-09-17 , DOI: 10.1021/acs.orglett.1c02856 Julian Simke 1 , Tom Bösking 1 , Bart Jan Ravoo 1
Affiliation
|
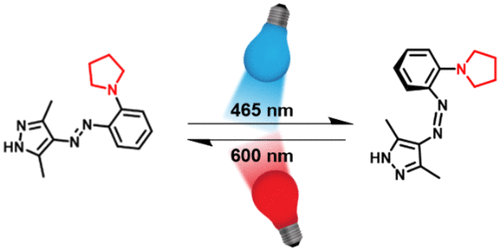
Bidirectional photoswitching of arylazopyrazoles with visible light is enabled by substitution with pyrrolidine and piperidine in the ortho-position of the phenyl ring. The absorption maxima were red-shifted and the molar absorption coefficients in the visible range increased significantly, allowing the use of blue light (λ = 465 nm) for the E → Z isomerization and red light (λ = 600 nm) for the Z → E isomerization. N-Methylation of the pyrazole leads to an excellent thermal stability of the Z isomer.
中文翻译:

红光对邻氨基芳基吡唑的光开关
通过在苯环的邻位用吡咯烷和哌啶取代,可以实现芳基唑并吡唑在可见光下的双向光开关。吸收最大值红移,可见光范围内的摩尔吸收系数显着增加,允许使用蓝光(λ = 465 nm)进行 E → Z 异构化和红光(λ = 600 nm)用于 Z → E 异构化。吡唑的N-甲基化导致 Z 异构体具有优异的热稳定性。
更新日期:2021-10-01
中文翻译:

红光对邻氨基芳基吡唑的光开关
通过在苯环的邻位用吡咯烷和哌啶取代,可以实现芳基唑并吡唑在可见光下的双向光开关。吸收最大值红移,可见光范围内的摩尔吸收系数显着增加,允许使用蓝光(λ = 465 nm)进行 E → Z 异构化和红光(λ = 600 nm)用于 Z → E 异构化。吡唑的N-甲基化导致 Z 异构体具有优异的热稳定性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号