当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Phase-Transfer Catalyzed Asymmetric [4 + 1] Annulations for the Synthesis of Chiral 2,2-Disubstituted Tetrahydrothiophenes
Organic Letters ( IF 5.2 ) Pub Date : 2021-09-16 , DOI: 10.1021/acs.orglett.1c02744 Qi Yin 1 , Xiaolu Wen 1 , Yiwei Chen 1 , Xiangnan Gong 2 , Lin Hu 1
Organic Letters ( IF 5.2 ) Pub Date : 2021-09-16 , DOI: 10.1021/acs.orglett.1c02744 Qi Yin 1 , Xiaolu Wen 1 , Yiwei Chen 1 , Xiangnan Gong 2 , Lin Hu 1
Affiliation
|
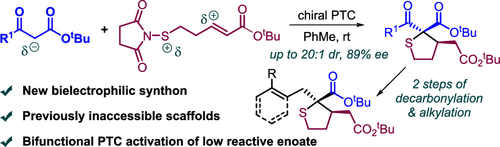
An efficient catalytic asymmetric [4 + 1] reaction, which features the use of simple β-keto esters as one-carbon nucleophiles and 5-succinimidothio-pent-2-enoates as four-atom bielectrophiles, has been developed in the presence of a bifunctional chiral phase-transfer catalyst. The new annulation provides a distinct protocol to access the functionalized 2-acyl-2-carboxyl tetrahydrothiophenes bearing consecutive quaternary and tertiary carbon stereocenters in high diastereoselectivities and enantioselectivities. Moreover, the prepared products could be readily transformed into the chiral 2-alkyl-2-carboxyl tetrahydrothiophenes via two steps of debenzoylation and alkylation reactions.
中文翻译:

用于合成手性 2,2-二取代四氢噻吩的相转移催化不对称 [4 + 1] 环化
一种高效的催化不对称 [4 + 1] 反应,其特点是使用简单的 β-酮酯作为单碳亲核试剂,使用 5-琥珀酰亚胺硫代-戊-2-烯酸酯作为四原子双亲电试剂,在双功能手性相转移催化剂。新的环化提供了一种独特的协议,以获取具有高非对映选择性和对映选择性的连续季和叔碳立体中心的官能化 2-酰基-2-羧基四氢噻吩。此外,制备的产物可以通过脱苯甲酰化和烷基化反应两个步骤容易地转化为手性 2-烷基-2-羧基四氢噻吩。
更新日期:2021-10-01
中文翻译:

用于合成手性 2,2-二取代四氢噻吩的相转移催化不对称 [4 + 1] 环化
一种高效的催化不对称 [4 + 1] 反应,其特点是使用简单的 β-酮酯作为单碳亲核试剂,使用 5-琥珀酰亚胺硫代-戊-2-烯酸酯作为四原子双亲电试剂,在双功能手性相转移催化剂。新的环化提供了一种独特的协议,以获取具有高非对映选择性和对映选择性的连续季和叔碳立体中心的官能化 2-酰基-2-羧基四氢噻吩。此外,制备的产物可以通过脱苯甲酰化和烷基化反应两个步骤容易地转化为手性 2-烷基-2-羧基四氢噻吩。



























 京公网安备 11010802027423号
京公网安备 11010802027423号